The amphibole supergroup is a group of more then hundred rock-forming silicates. This article provides general introduction to structure, physical properties and chemical composition of amphiboles.
What are amphiboles?
All amphibole mineral species have perfect cleavage in two directions and a splintery fracture. Their color typically is dark green, brown or black, although many colors, including colorless, white, yellow, green, blue, and lilac are known. Amphiboles occur in both metamorphic and igneous rocks, frequently as dark elongated grains and crystals embedded in the rock, but can occasionally form well developed crystals. Such crystals are most commonly found in pegmatites and as porphyroblasts in igneous rocks, marbles/skarns and veins within metamorphic rocks.

Bright green pargasite in marble from Luc Yen, Yen Bai Province, Vietnam. Size: 7 x 5 cm; photo:
Parent GéryThe dark color, crystal form, hardness, and well-developed cleavage usually serve to distinguish these minerals from other common rock-forming minerals. The pyroxene group can appear in similar environments and has physical characteristics similar to the amphiboles: the two mineral groups can be distinguished based on the angles between their cleavage planes; the amphiboles have cleavage planes with 56˚ and 124˚ and the pyroxenes have 87˚ and 93˚. Amphiboles can also be found as pseudomorphs after pyroxenes, often making the distinction even harder. Amphibole crystals can also be identified by their six-sided crystal cross sections.
Amphibole complexity
The amphibole group minerals are generally considered amongst the most complex silicate groups. There are several reasons for this, but the basis for the complexity is the large chemical variation within the same molecular structure, resulting in a wide variety of mineral species with similar physical properties.
The chemical variation is expressed by Hawthorne and Oberti (2006):
The chemical composition and variability of the amphiboles may be expressed by the general formula AB2C5T8O22W2, where:
- A = □, Na, K, Ca, Pb2+
- B = Li, Na, Mg, Fe2+, Mn2+, Ca
- C = Li, Mg, Fe2+, Mn2+, Zn, Co, Ni, Al, Fe3+, Cr3+, Mn3+, V3+, Ti4+, Zr
- T = Si, Al, Ti4+
- W = (OH), F, Cl, O
There are more than 100 minerals approved by the IMA as a result of this chemical variability. There are probably several tens of additional species identified, but yet to be properly described. The chemical diversity also shows within a single grain or crystal. Zoning is common within a single crystal and a locality may well contain 5 or more species. Mont St-Hilaire in Canada, is extreme in this respect as up to 10 different amphibole species with arfvedsonite root names may be present, some of which are not (yet) properly described.

Classic Alpine actinolite schist (dark green needle shaped crystals) from Habachtal, Austria. Size: 10 x 7 cm; photo: Zbyněk Buřival
The acknowledged amphibole nomenclature use defined rules of prefixes to distinguish between closely related species, giving names like potassic-magnesio-arfvedsonite and ferro-ferri-taramite. Such naming conventions are very useful in showing how closely related species relate, but can also be confusing. For example, sometimes the mineral name, such as arfvedsonite is used synonymously with the root-name arfvedsonite so it can be hard to know whether the author refers to a specific mineral or the more general root-name.
The nomenclature also allows a category of so-called named amphiboles. These are amphiboles that have been identified via Electron Micro Probe Analyzer (EMPA) analysis, but are not properly described and thus not approved by the CNMMN (Committee of New Minerals and Mineral Names, which is one of the committees under the International Mineral Association - IMA).

Aggregate of the phlogopite mica (inner part) and fibrous anthophyllite (pale fibrous rim) from Heřmanov, Czech Republic. Size: 8 x 8 cm; photo: Zbyněk Buřival
The principles for assigning a name to a named amphibole are the same as for approved minerals. The amphibole ferro-chloro-pargasite, has been identified from Lukkulaisvaara in Karelia, Russia and published as a named amphibole. It is not possible to see whether this is an approved amphibole mineral or simply a named amphibole based on the name alone. One has to look up in the IMA list of approved minerals to make sure.

Pale crystals of tremolite from Campolungo, Leventina, Kanton Tessin, Switzerland. Size: 14 x 6.5 cm; photo:
Didier DescouensBecause of the complex chemistry (and the resulting complex nomenclature), amphiboles require careful and sophisticated analytical work to be properly characterized and identified. The most common and inexpensive techniques for analysis amongst collectors, EDS (Energy Dispersive X-ray Spectroscopy), WDS (Wave-length Dispersive X-ray Spectroscopy) and XRD (X-ray Powder Diffraction) are only suitable for approximate identification of most amphibole compositions, although they can be helpful for distinguishing some of the near-end member compositions. EDS/WDS as well as XRD will easily distinguish between anthophyllite and tremolite from a mafic rock, and it may give approximate identifications indicating that a certain amphibole is hastingsitic or richteritic, these techniques cannot be used to separate magnesio-hastingsite from ferro-hornblende to state an example.

Eudyalite (red) with arfvedsonite (black) from Kipawa River, Villedieu Township, Quebec, Canada. Size: 7 x 6 cm; photo:
James St. JohnEven the most commonly used method amongst scientists, the Electron Micro Probe Analyzer (EMPA) can provide inaccurate results. EMPA does not analyse for H or Li, which both can be important for identification of the species. Other important elements such as F, Cl, Mn and others are often omitted from the analysis, giving slightly distorted normalized formulas that can lead to mis-identification. This creates uncertainty in the identification of minerals near the borderline of a solid solution series, or in other words most naturally occurring amphiboles. However, the greater problem is that EMPA does not distinguish between Fe2+ and Fe3+, which is very important for identification of most amphibole species. This ratio is normally calculated, and good spreadsheets are available (Locock 2014), but the results are inaccurate and may lead to inaccurate identification. Accurate identification of an amphibole requires a combination of methods, such as XRD, EMPA, LA-ICP-MS and Mössbauer spectroscopy, but the complexity of absolute identification means that this is rarely done. Atlases of XRD, IR and Raman spectra for amphiboles does not get any better than the original identification of the samples used for such tables, and are consequently of limited use.

Fibers of dark ferro-papikeite in sekaninaite from Dolní Bory pegmatite, Czech Republic. Size: 2 x 1.5 cm; photo: Zbyněk Buřival
Although the group is certainly complex, there is no reason to give up on it. As collectors, we need to spend some time studying the group, and we must accept the fact that it will not always be practical or even possible to identify the correct specie(s) contained in a specimen. Personally, I label amphiboles with what I consider reasonable certainty. For tremolite and other common amphiboles, and amphiboles that are dominant at their locality, I can label a specimen with a species name. More exotic amphiboles or amphiboles from poorly described localities or with chemistry that is hard to analyze I often label a specimen amphibole or use adjectival modifiers as recommended by the amphibole sub-committee of the CNMMN. I find nothing wrong with the terms kearsutitic amphibole or barrositic amphibole in cases where I have specific amphiboles from a known locality but lack sufficient analytic evidence.

Blue glaucophane with green fuchsite mica from Groix Island, Morbihan, Brittany, France. Size: 6.5 x 5 cm; photo:
Didier DescouensAny exotic amphiboles offered for sale should be accompanied with proper analytical data or at least literature reference for the locality allowing the buyer/collector to judge the likelihood of an identification. Otherwise any identification should be treated with high caution.
The Amphibole Atomic Structure: The Double Chain
The complexity and flexibility of the amphibole molecule is the root cause for the complexity of the amphiboles. The basic building block for the amphiboles is the same SiO4 tetrahedra that are the building block and core of all silicates. In the amphiboles, these tetrahedra form long double chains:
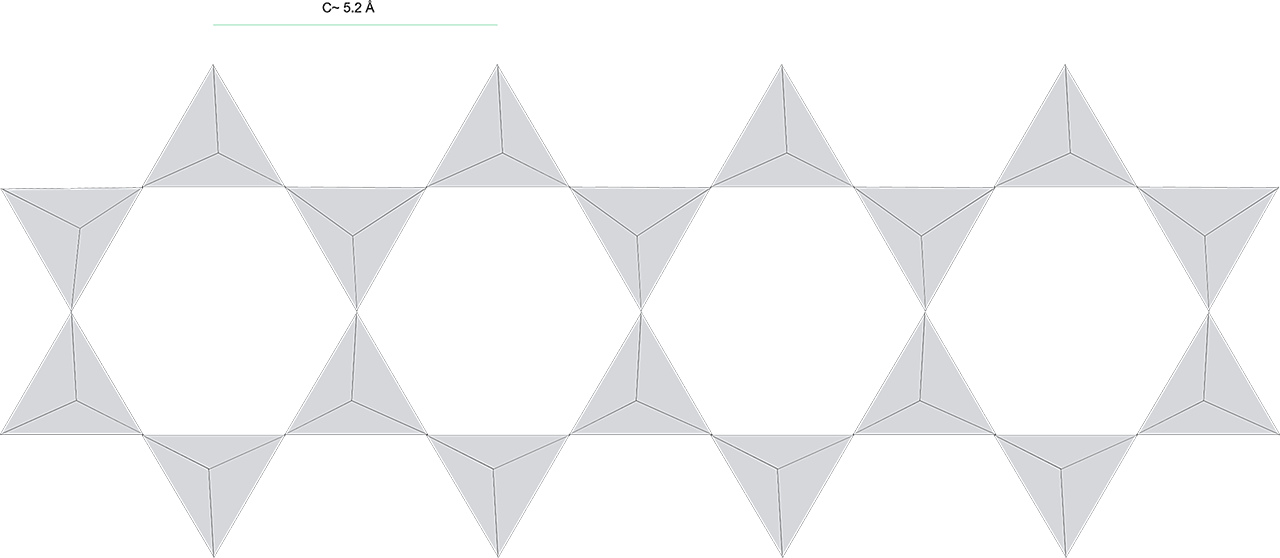
Figure 1: Theoretical representation of the double chain structure of the amphiboles, as formed by the SiO4 tetrahedra.
Between and associated with these chains, other elements find their space. Figure 2 shows an approximate representation of the positions of these other elements, which in this case constitute the generic formula of amphibole: AB2C5T8O22W2.
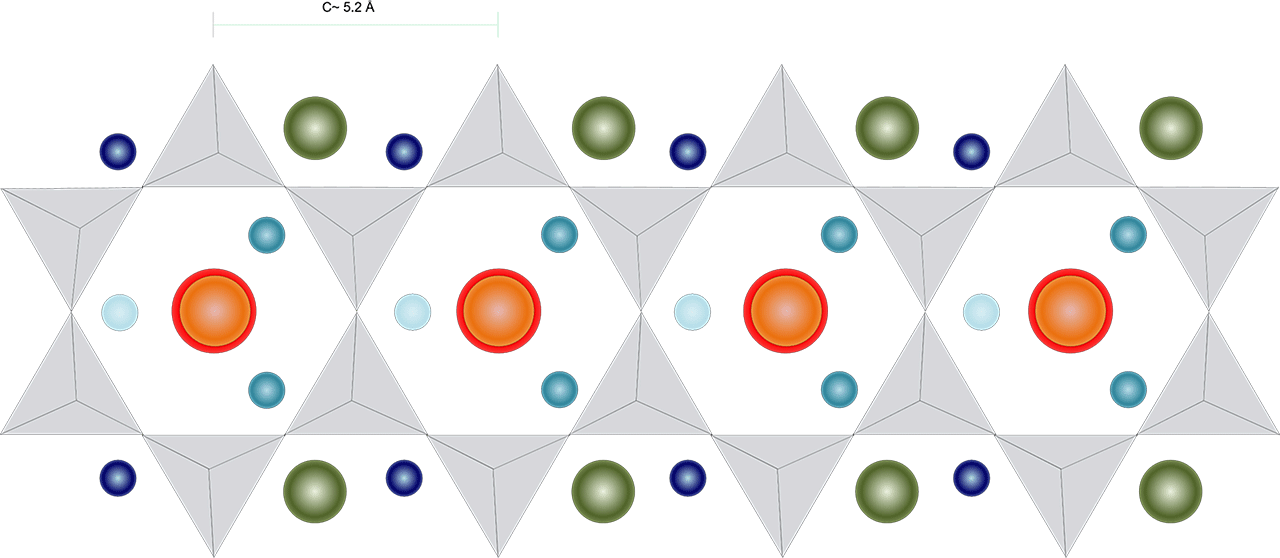
Figure 2: A theoretical representation of the amphibole double chain with additional elements
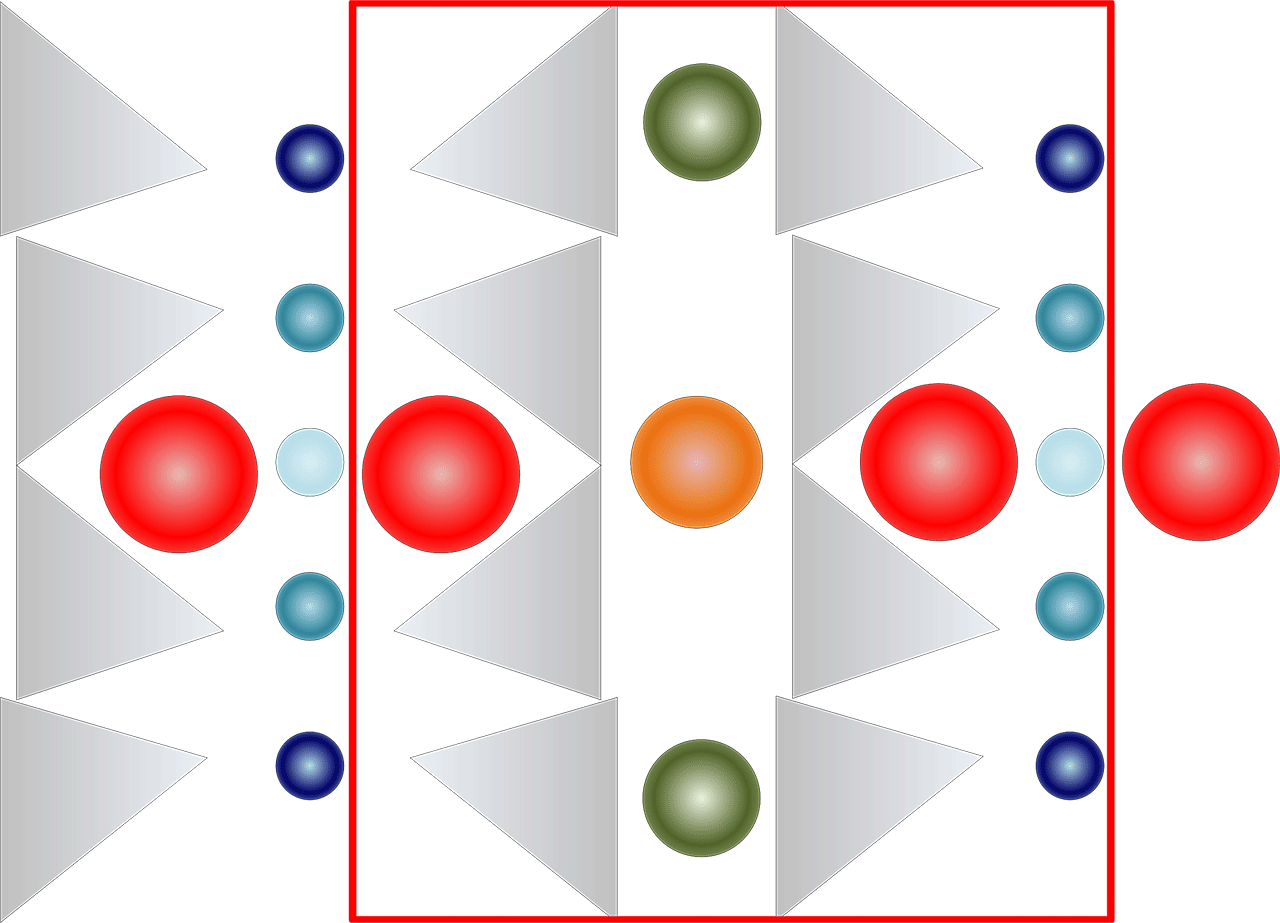
Figure 3: A theoretical representation of the amphibole molecule viewed along the double chains, showing the relative position of the elements in the formula. The red square represents a formula unit (AB2C5T8O22(OH)2
In both figure 2 and 3, the circles represent the position and an approximate size for typical ions filling the voids between the double chains as represented in the general formula of amphibole.
- The orange sphere represents the A position, shown with a size roughly similar to the relative size of Na.
- The green spheres represents the B position, (also called M4), shown with a size roughly similar to the relative size of Ca.
- The different shades of blue represent the C position (also called M1 to M3.), are shown with a size roughly similar to the relative size of Mg.
- The grey triangles represent the SiO4 tetrahedra, the T8O22 part of the formula. Often, some Si is replaced by Al.
- Red spheres represent the W position, which is normally mostly (OH), but can also be F, Cl and O. Their size is roughly similar to the relative size of (OH).
It is the flexibility of the double chains that results in the chemical complexity of the amphiboles. The chains can be distorted, thus allowing ions of different size and charge to fill the different voids. Figure 4 illustrates some of this flexibility by an example given by Heaveysege et al. (2015). Compared to the ideal double chain presented in figure 1, this is clearly distorted, allowing different elements to fill different positions.
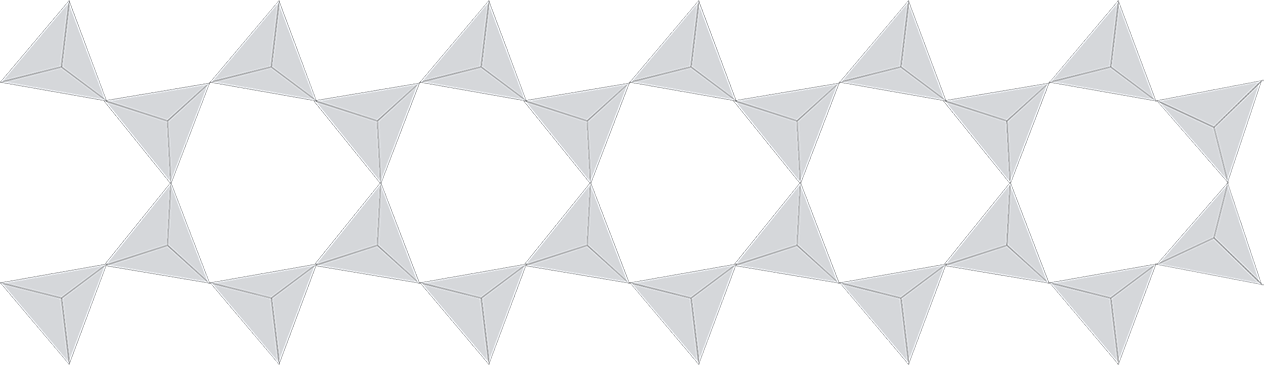
Figure 4: Slightly distorted double chain structure of pargasite from Myanmar as presented by Heaveysege et al. (2015)
The Example of Tremolite and Arfvedsonite
Tremolite, □Ca2Mg5Si8O22(OH)2 and NaNa2Fe2+4Fe3+Si8O22(OH)2 are both amongst the more common amphiboles. Looking at the chemical formula, the similarity with the general amphibole AB2C5T8O22W2 can easily be seen, yet the chemistry of the two amphibole species are quite different:
| Amphibole species | A position | B position | C position | T position | W position |
|---|
| Tremolite | Vacant | Ca2 | Mg5 | Si8 | (OH)2 |
| Arfvedsonite | Na | Na2 | Fe2+4Fe3+ | Si8 | (OH)2 |
Figure 5: A two-dimensional representation of the calcic amphiboles in terms of the edenite and tschermak exchange vectors.
I find the use of exchange vectors a very useful tool in explaining the relationship between the different amphiboles. I also use it as an approximate guide in assessing the rarity of an amphibole and also in considering the likelihood of amphiboles occurring together.
Zingg (1996) have plotted actual amphibole compositions in vector plots, showing good correspondence between these rules and amphibole compositions found in nature.
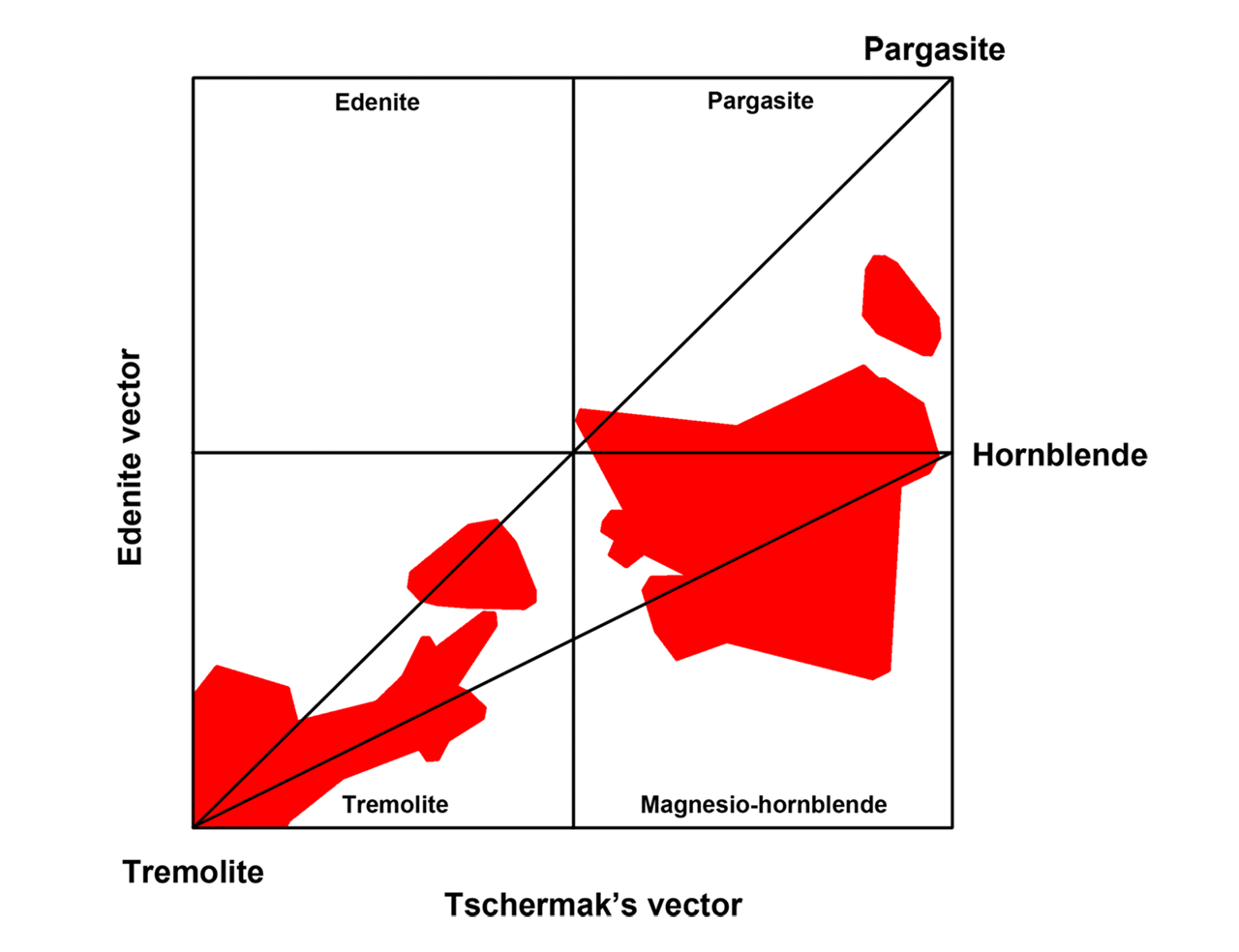
Figure 6: A modified plot originally compiled by Zingg (1996), showing amphibole compositions plotted along tschermak and edenite exchange vectors, showing compositional pairs of calcium-amphiboles. The usefulness of these vectors in representing compositions are obvious. From this plot, the miscibility gap between tremolite and magnesio-hornblende/pargasite are evident, as is an approximate 3:2 ratio between the tschermak and edenite vectors.
These exchange vectors are also important in understanding and explaining the occurrence of different amphiboles in nature. An example is the retrograde metamorphism of blueschist and eclogite rocks. These are rocks that are formed deep in the crust at high pressures and, for blueschist, relatively low temperatures. During the normalization of PT conditions and uplift through the crust, blueschist is often fully or partially transformed via retrograde metamorphosis to greenschist or amphibolite facies rocks, i.e the sodic amphibole glaucophane [□Na2(Mg3Al2)Si8O22(OH)2] becomes actinolite [□Ca2(Mg4Fe)Si8O22(OH)2], magnesio-hornblende [□Ca2(Mg4Al)AlSi7O22(OH)2] or pargasite [NaCa2(Mg4Al)Al2Si6O22(OH)2] respectively.
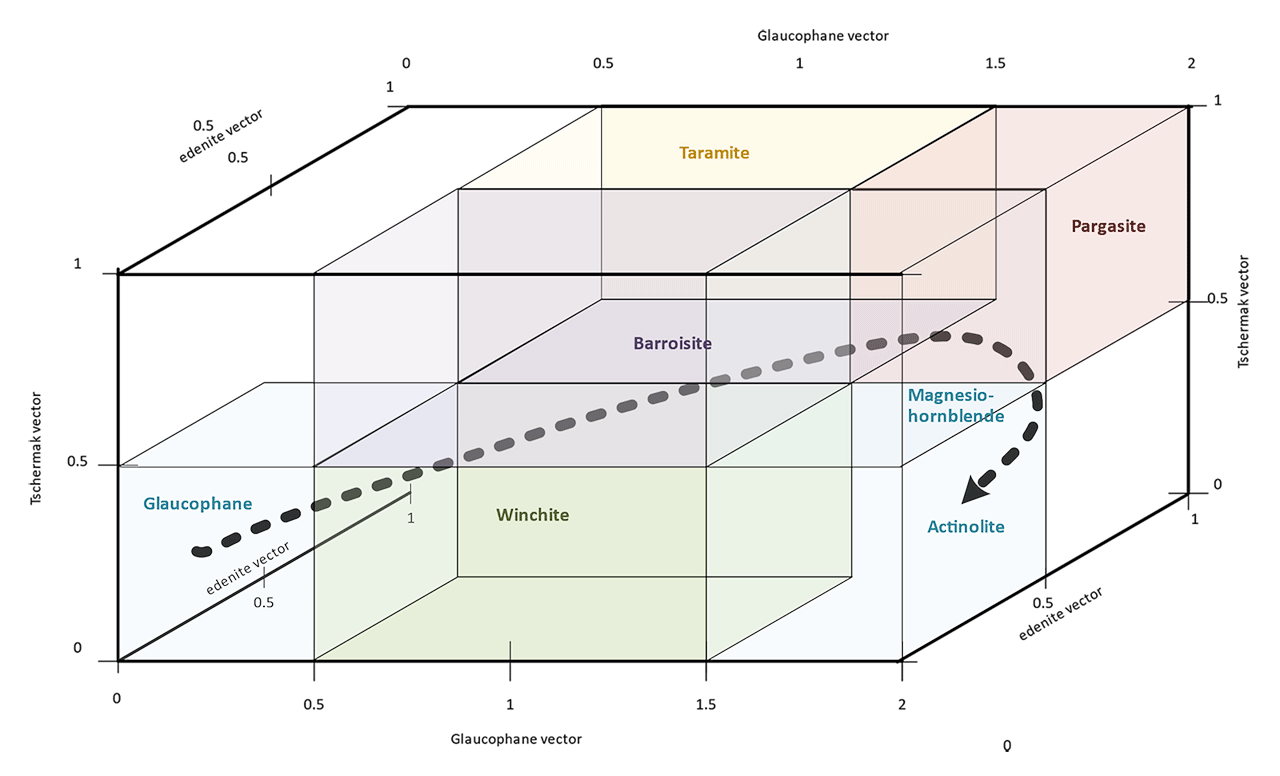
Figure 7: The relationship between glaucophane and the calcic amphiboles as found in retrograde blueschists and eclogites.
The figure 7 shows relationship between glaucophane and calcic amphiboles expressed via exchange vectors. The dotted arrow indicate a possible, theoretical exchange path during retrograde metamorphosis. Starting from glaucophane in a blueschist, the amphiboles in an amphibolite facies rock will normally be in the magnesio-hornblende/pargasite compositional space, whereas actinolite will be the dominant amphibole in a greenschist facies rock. It should be no surprise that barroisite, taramite and winchite can be found as grains, rims or zones in such rocks.

Tremolite crystals from Wilberforce, Haliburton Co., Ontario, Canada. Size: 6 x 5 cm; photo:
Didier DescouensAmphibole Mineral Species Definitions
Generally, but not without exceptions, every amphibole that can be derived from another with an exchange vector/coupled substitution gets a unique root-name. Examples of different root names can be seen in figures 5 and 6. Important exceptions to this general rule are:
- pargasite and hastingsite that are related via an Al <=> Fe3+ substitution
- tremolite and actinolite that are related via a Mg <=>Fe2+ substitution
- eckermannite and arfvedsonite that are related via a MgAl <=> Fe2+Fe3+ substitution
These and other such exceptions are kept (grandfathered) as a result of their common usage, both in mineralogy and petrology. Making these exceptions maintain the traceability of in the literature and descriptions of the minerals, and to a certain degree to retain the historical significance for these mineral names.
Element by element substitutions with the same root-name create new mineral species. Pargasite root-name amphiboles are listed as an example below:
| Mineral specie | Formula | IMA status |
|---|
| Chromio-pargasite | NaCa2(Mg4Cr3+)(Al2Si6O22)(OH)2 | Approved |
| Ferro-chloro-pargasite | NaCa2(Fe2+4Al)(Al2Si6O22)Cl2 | Named |
| Ferro-pargasite | NaCa2(Fe2+4Al)(Al2Si6O22)(OH)2 | Approved |
| Fluoro-pargasite | NaCa2(Mg4Al)(Al2Si6O22)F2 | Approved |
| Pargasite | NaCa2(Mg4Al)(Al2Si6O22)(OH)2 | Approved |
| Potassic-chloro-pargasite | KCa2(Mg4Al)(Al2Si6O22)Cl2 | Approved |
| Potassic-ferro-pargasite | KCa2(Fe2+4Al)(Al2Si6O22)(OH)2 | Approved |
| Potassic-fluoro-pargasite | KCa2(Mg4Al)(Al2Si6O22)F2 | Approved |
| Potassic-pargasite | KCa2(Mg4Al)(Al2Si6O22)(OH)2 | Approved |
It is important to realize that all of these minerals are part of a solid solution series, so that for each of them, all of the element by element substitutions can be present to some degree. In addition some Al <=> Fe3+ substitution and one or more coupled substitutions may be involved. The empirical pargasite formula listed earlier is a good illustration in that respect. Near end-member amphiboles are not common and any name and normalized formula is somewhat arbitrary.
To identify any of these pargasite minerals with some confidence require at a minimum an EMPA analysis. Taking into consideration both F, Cl, Ti, and other ions that may be present in the C position is important. Even then, the species designation requires a calculation of Fe2+/Fe3+ ratio, H and Li content. Consequently, borderline compositions may well be incorrectly named, even when supported by analytical data and correctly normalized.

Green actinolite crystals in talc from Habachtal, Austria. Size: 7 x 6 cm; photo: Zbyněk Buřival
Therefore, it is recommended for all of us to relax our desire to have every mineral named in accordance with IMA’s monthly list of minerals. Even the IMA amphibole nomenclature encourages scientists to use adjective modifiers, naming a specimen pargasitic amphibole if literature studies or microprobe (EDS/WDS) data indicates an Al and Ca rich amphibole corresponding to one of the pargasite mineral species. This practice is highly recommended, as it at the same time provides a correct label without guessing too much, and it also limits the compositional space of your specimen so that other people get a good understanding on what kind of amphibole you have.
Common Amphiboles
There are more than a 100 amphibole species, most of them very rare and very difficult to identify. There are however a few common amphiboles that are likely to occur in large well formed crystals or aggregates. Most of these can be distinguished by their petrologic setting, appearance or approximate chemical composition. These are:
| Name | Chemical composition | Typical appearance* | Typical environment* |
|---|
| Tremolite | □Ca2(Mg5)(Si8O22)(OH)2 | Light colored crystals or aggregates | Carbonate rocks and calcic mafic rocks |
| Actinolite | □Ca2(Mg4Fe)(Si8O22)(OH)2 | Green crystals or fibrous aggregates, byssolite variety | Talc schists and greenschists, Alpine fissures |
| Hornblende
(Magnesio-hornblende to pargasite) | □Ca2(Mg4Al)(AlSi7O22)(OH)2 to NaCa2(Mg4Al)(Al2Si6O22)(OH)2 |
Black masses or individual crystals | Amphibolites, skarn and other metamorphic rocks. Basalts and related rocks |
| Magnesio-arfvedsonite | NaNa2(Mg4Fe3+)(Si8O22)(OH)2 | Dark green to black crystals / byssolite | Pegmatites and other igneous rocks |
| Riebeckite | □Na2(Fe2+3Fe3+2)(Si8O22)(OH)2 | Dark blue to black crystals / crocidolite | Pegmatites and other igneous rocks |
| Anthophyllite | □Mg2(Mg5)(Si8O22)(OH)2 | Typical light brown fibrous aggregates | Mafic and ultra-mafic rocks, and in ortho-amphibole – cordierite gneisses with gedrite |
| Cummingtonite | □Mg2(Mg5)(Si8O22)(OH)2 | Typical light brown fibrous aggregates | Mafic rocks and non-Fe metal ores |
| Glaucophane | □Na2(Mg3Al2)(Si8O22)(OH)2 | Blue schistose aggregates | Blueschists |
* The “typical” colours and environments are just that. Tremolite may be white, brown, lilac, green, blue and yellowish hues, and hornblende can be both green and brown. Pitch-black actinolite is known to occur in high grade amphibolites and skarns in Southern Norway. The information in these columns is included so that an average collector may get an approximate ID on self collected amphiboles. A green amphibole associated with talc can safely be labelled actinolite and a brown amphibole associated with cordierite is safe to assume as belonging to the anthophyllite-gedrite series, but there are few absolutes in the world of amphiboles.

Blue fibrous riebeckite (variety riebeckite - amphibole asbestos) from South Africa. Size: 3 x 2 cm; photo:
Siim SeppThe vast majority of amphibole specimens belongs to one of these root-name compositions, although other species may be locally abundant. This table provides a first pass identification chart for amphiboles. Most of these amphiboles can be tentatively identified visually or by semi-quantitative EDS or XRD analysis. Any unusual results, not near any of these compositions may warrant further studies, either by literature studies, EMPA or a combination of methods. Also feel free to use these names as adjectives, such as sodic amphibole, anthophyllite-gedrite series, actinolitic amphibole on the labels, even though arfvedsonitic or glaucophanic amphibole is quite a mouthful.

Bluish crystals of sodalite (var. hackmanite) in pale winchite matrix from Kokcha Valley, Badakhshan Province, Afghanistan. Photo:
Parent GéryAny amphibole listed for sale containing fluoro-, chloro- or oxo- prefixes should be treated with suspicion. The quantity of the elements (F, Cl and H) required to assign such prefixes are often not correctly determined. Even EMPA can not analyse H (H2O) content and older EMPA models (like Cameca SX50) have also high error margin for measuring F and Cl. The same goes for amphiboles containing Li as a required constituent in the formula. EMPA can not analyse Li, special methods like LA-ICP-MS or AAS must be used. Of course such amphiboles exist, but analytical data or solid literature references should accompany such amphiboles.
Also be careful with Al-bearing sodic-calcic amphiboles ( winchites, taramites, barroisites etc.) as these rarely occurs as anything but very small grains and often only as zones in these grains. Also NaMn or NaMg amphiboles (such as ghoseite) as well as sodic-anthophyllites and gedrites are very rare. As the Na and Mg/Mn ions are placed next to each other in the structure for these amphiboles, the size differences between the Na ion and the Mg/Mn ions impacts the stability of these amphiboles.

Green pargasite crystals in calcite from Hunza Valley, Gilgit-Baltistan, Pakistan. Photo:
Parent GéryEdenite and tschermakite end-member compositions are unstable. Fe2+ and F aid the stability of edenite, and many edenites are in reality fluoro-edenite or ferro-edenite. The occurrence of edenite from its type locality Edenville, USA is questionable. Most tshcermakites have a composition close to the more stable pargasite, and have substantial Fe3+ replacement for Al. Both of these conditions tend to somewhat stabilize this mineral.
Summary
The amphibole supergroup is certainly complex in that it shows a wide chemical diversity amongst minerals with very similar physical properties, such as color, cleavage, crystal habit and hardness. Even within the same specimen - or even crystal - multiple amphibole species can be present. As a result, scientists have had difficulties in finding a nomenclature that encompasses the natural variability of amphibole compositions. Frequent nomenclature changes and redefinitions does not make understanding of the amphibole supergroup any easier. Also, standard analytical techniques often fall short in accurately determining the species present in a specimen or a locality. We all like a mineral name on our labels, but we are often too optimistic when naming amphiboles. Consequently, many (most?) of the rare amphiboles are misidentified.

Pseudomorph of limonite after riebeckite from South Africa. These pseudomorphs are composed of iron oxides and quartz, often labeled as crocidolite (fibrous riebeckite), but in fact the original amphibole is (almost) completely decomposed. Size: 6 x 3.5 cm; photo: Zbyněk Buřival
However, spending a little time to understand the amphibole structure and the distribution of ions between the different sites can give a basic understanding of the few main building blocks for the wide variety of amphibole species. An understanding of the primary substitutions and/or exchange vectors will also help in decoding this complex group of minerals. Understanding or accepting the more common exchange vectors can also give the key to understanding the amphibole nomenclature.
A more thorough study of the environments in which the different amphibole species belong can also give valuable insight in petrology and the geologic processes that forms minerals. It is my hope that this article may give some insight in the, despite all, fascinating minerals.
References
- Heaveysege, D., Abdu, Y. A., & Hawthorne, F. C. (2015). LONG-RANGE AND SHORT-RANGE ORDER IN GEM PARGASITE FROM MYANMAR: CRYSTAL-STRUCTURE REFINEMENT AND INFRARED SPECTROSCOPY. The Canadian Mineralogist, 53(3), 497-510.
- Hawthorne, F. C., & Oberti, R. (2006). On the classification of amphiboles. The Canadian Mineralogist, 44(1), 1-21.
- Pasero. Marco (2017) The New IMA List of Minerals – A Work in Progress – Updated: March 2017
- Zingg, A. J. (1996). Immiscibility in Ca-amphiboles. Journal of Petrology, 37(3), 471-496.
- Deer, W.A., Howie, R.A, Zussmann, J. (1997): Rock-forming Minerals: Double-Chain Silicates, Volume 2B, second edition.
- Revheim, O., Currier, R., Gyllenhammer, R.J. (2012) Arfvedsonite. revision 1.0. Mindat Best Minerals Project, article "mesg-66-138948
























Comments