Diamond - the extreme King of Gems
Diamonds are forever, and they are a girl's best friend, but as minerals go they are not just another pretty face. Noted from ancient times for beauty, brawn and mystery - and today for impacting a broad segment of modern life - diamonds stand out as one of the most exceptional of all minerals.
Beauty
Most people equate diamonds with the cut and polished gemstones used in jewelry - usually of the most expensive and extravagant type. Celebrities from rock stars to royalty use diamonds to flaunt their wealth and position. Legends - often tragic ones - develop around diamonds, and families split over who gets a deceased relative's diamond engagement ring.

All this glitter comes at a price: with the possible exception of gold, diamond incites more greed than any other mineral. In Africa, for example, thousands of people have been murdered or maimed by more powerful and better-armed groups seeking to seize control of their mines.
Brawn
Diamond also has less glamorous yet critical applications as an industrial mineral. Diamonds - often synthetic - are used in drill bits, saws, and grinders, enabling us to drill, cut, and grind materials that were previously unworkable.
Mystery of Origin
As mentioned below, the origin of diamonds is a subject of debate. The search for those factors connecting diamonds in Canada to those in Africa remains intense to this day.
Diamond Overview
The name diamond can trace its origins to the Greek adamas, Latin adamans, meaning unbreakable and Medieval Latin, diamas/diamant. The old French diamant was favored until the English word adamant was introduced. The current name diamond originates in Middle English.
Diamond is a cubic allotrope of carbon, other being hexagonal graphite and hexagonal lonsdaleite. Diamond is part of the class of non-metallic elements.

Right: Octahedral 3 mm diamond crystal from river sediments in Sakha Republic, Russia. Photo: Vítězslav Snášel
Almost all diamonds are extremely old (dated to between 1-3.3 billion years). Although they are primarily found in volcanic deposits known as pipes, or in alluvial deposits produced by the erosion of these pipes, their origin is still quite uncertain. Exceptionally, small diamonds can be formed during meteorite impacts.
The largest diamond ever found is the carbonado Sergio, weighing in at 3167 carats (rough). A carbonado (dark) diamond, it was found in Bahia, Brazil in 1895. The largest gem quality diamond is the Cullinan, at 3106 carats (rough), found in Pretoria mine, South Africa in 1905.
Physical Properties of Diamond
In the forum of material properties, few other minerals can outstrip the diamond. It is the hardest known natural material, and its structure is the densest of all known natural materials. Diamonds are very resistant to both acids and bases. In addition, it is the most efficient heat conductor, has the highest melting point of any substance (4090 °C), and the highest refractive index of any natural mineral. Diamonds are natural electrical insulators, although the presence of impurities such as boron may result in semiconductor properties.
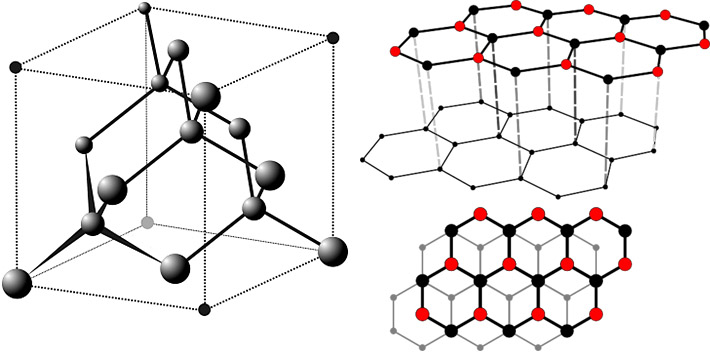
The diamond is formed by tetrahedrally coordinated carbon atoms in a covalent network lattice (sp3) - called diamond lattice. This means that each carbon atom is surrounded by 4 other atoms. Diamonds have dense isometric structure, isostructural with sphalerite. They form octahedral or less likely cubic crystals and readily form twins. Twins usually have many crystal faces, which are often rounded and crystals can be almost round. The specific gravity of diamond is about 3.5-3.6 g/cm3.
The diamond is the hardest mineral on Mohs scale (10). In spite of this hardness, diamonds show very good octahedral cleavage and are fairly brittle. Some diamonds with lot of inclusions like bort or carbonado show slightly reduced hardness and can even lack cleavage. Diamonds are extremely pressure resistant: they will not break up to 600 GPa.

Rough diamonds have a greasy luster; processed diamonds have an adamantine luster, defined as a very high refractive index, displaying brilliance and shine. Some may even show UV fluorescence.
Chemically pure diamonds are clear, and are relatively rare. Diamonds containing impurities or structural defects resulting in changes to their color are much more common. In the case of diamonds, the only impurities found in the crystal lattice of a diamond are nitrogen, hydrogen and boron. The most common color of diamond is yellow and brown, followed by colorless and then by various colors (fancy diamonds). Nitrogen is most common impurity in diamond structure and causes light yellow to light brown color. Boron is much more rare and results in blue color. Irradiation causes green color and plastic deformation of the crystal structure causes brown and probably also pink and red color. An excess of dark impurities results in the dark color of carbonado diamonds.
Origin of Diamonds
The process of diamond formation is still not well understood. According to traditional theory, conditions including require pressures of 4.5-6 GPa and temperatures between 900-1300 °C must be present. These conditions exist only in the Earth's mantle with a proper thermal gradient - and can only be met under continental crust at depths about 140-190 km.

Right: Diamond crystal 3 mm big on kimberlite matrix, Kimberley, South Africa.
Both photos: Zbyněk Buřival, collection of Moravske Zemske Muzeum.
Stable isotope tracing reveals two possible sources for the carbon required for the formation of diamonds. So called harzburgitic diamonds are found in mantle rock that is inorganic in origin, while eclogitic diamonds, formed by subduction of litosphere with organic sediments deep into the mantle can contain carbon of both inorganic and organic origin.
The theory states that diamonds are transported to the surface by the volcanic eruptions originating at extreme depth, and here it breaks down: the mechanism of such eruptions is still murky. Magma containing diamonds pierces the crust and formes a volcanic channel called pipe. These pipes often contain volcanic breccia with many crust and mantle rocks xenoliths (pieces of rocks incorporated into the magma). These rocks are known as kimberlites or lamproites. Diamonds are carried within mantle rock xenoliths which later decompose into serpentinite minerals during hydration and decompression.

The theory above has some flaws though. It fails to account for the occurrence of microscopic diamonds alongside garnets and kyanites in some granulite rocks which were formed in an environment that is much shallower than the upper mantle. Other factors called into question include the extreme depth of subduction necessary to begin this process and its subsequent eruption from these depths.
Appropriately the most mysterious are the dark carbonado diamonds. Lacking all signs of having been formed either in the mantle or by meteorite impact, the origin of their enormous size remains a subject for debate.
Diamond Mining
Evidence points to the first diamonds having been discovered in India's Krishna River delta about 900 BCE, although some historians postulate that they were discovered far earlier. The first deposits discovered outside of India were found in Brazil in 1725; the first primary deposits of South African diamonds were discovered in 1870. Diamond production has since skyrocketed in last two centuries and has accelerated in the last few decades. Annual production of natural diamonds is currently around 25 tons.
Diamond production is controlled by a strong and interconnected network of mining companies and markets. The supply of gem-quality diamonds is controlled by creating artificial scarcity, and then lower-quality diamonds are sometimes used to satisfy the subsequent demand.

The mining of diamonds in conflict countries in central Africa, including Congo, represents an enormous human rights crisis, all in the name of profit. The military groups controlling these operations are well-known for employing extremelly unethical mining methods. They then sell the diamonds - referred to as blood diamonds - to big corporations to fund their wars. The diamond tracking procedure known as The Kimberley Process was introduced in 2002 to limit the introduction of these diamonds into the market, but it is still extremely problematic. Canada later introduced much more strict protocol to control the both the production and market value of diamonds mined in their territory.
Synthetic Diamonds
The 1879 discovery that diamond is composed of pure carbon initiated the search for ways to convert cheap forms of carbon (such as coal) into diamonds. The most important researchers at that time were James Ballantyne Hannay in 1879 and Ferdinand Frédéric Henri Moissan in 1893. Later, Sir Charles Algernon Parsons attempted to reproduce their experiments, as well as his own methods, with no success. Despite many wild claims, the first confirmed and reproducible diamond synthesis was achieved in Sweden in 1953 and in the USA in 1954. Diamond synthesis research was closely guarded for decades and many research secrets have yet to be revealed.
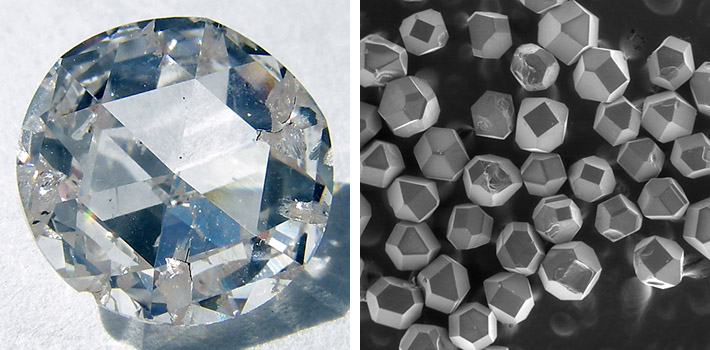
Right: About 0.3 mm large synthetic diamonds, scanning electron microscope image. Photo: Ludvig14
The current menthods include high-pressure high-temperature (HPHT), chemical vapor deposition (CVD) and lately also detonation synthesis and graphite treatment with high-power ultrasound. The first two methods are widely used in industrial production of synthetic diamonds and both can produce also gem grade diamonds. Detonation synthesis is used to produce powder diamond abrasives since 2000. Last ultrasound method is very new and has no industrial application yet.
Industrial Use of Diamonds
Industrial use does not require the quality and clarity of gem diamonds, which constitute only about 20 % of those found. The other 80 % are employed industrially, making use of diamonds' extreme hardness and thermal conductivity. Most industrial diamonds are used for cutting and grinding. The lowest grade diamonds (bort) are used as abrasive powder or incorporated into various tools ranging from construction and machinery tools to special diamond-tipped surgical scalpels.
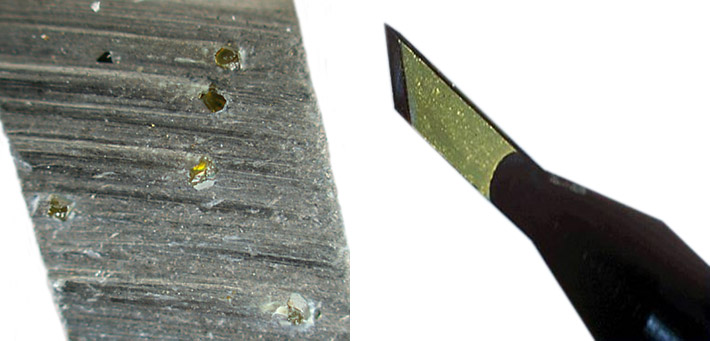
Right: Special diamond scalpel for presice medical operations. Photo: NIMSoffice
Diamonds also have high-tech applications. These include: the construction of diamond anvil-cells; extremely durable diamond bearings in special mechanics; heat sinks in modern electronics; diamond windows in X-ray or laser machines; diamond speaker domes; high-power switches; field-effect transistors; and light-emitting diodes.
Skyrocketing demand for industrial diamonds has resulted in an increase of synthetic diamond production from roughly 100 tons in 2004 to more then 900 tons in 2014. China produces about 90 % of all synthetic diamonds.
Gemmy Diamonds
Diamond is probably best known of the gem stones. The quality of gem diamonds is defined by 4C's - Color, Cut, Clarity and Carat. These basically define the color of the stone, quality and shape of its cut, clarity and number of inclusions and impurities and its weight in carats (1 carat = 0.2 g).
The most famous round brilliant cut was designed by mathematician Marcel Tolkowsky: the modern form of this cut has 57 facets. The shape was designed to reflect maximum amount of light and enhance the internal fire of the gem. There are also many other cuts used to create similarly pleasing effects.

Gem quality diamonds can be produced also by lab synthesis, however their price (40-50x more then natural gem diamonds) is prohibitively high. Synthetic gem-quality diamonds are very difficult to identify and can be easily treated to create colored fancy diamonds. Fake fancy diamonds are a big issue for the gem trade, prompting the development of special methods to confirm the origin of these extremely pricy gems.
The Biggest Gem Diamonds Ever Found
The biggest found gem grade diamond is 3106.75 carat Cullinan, which was found on 26 January 1905, in the Premier No. 2 mine, near Pretoria, South Africa. It was cut into 105 stones. The biggest one is Cullinan I (Great Star of Africa) weighting 530.2 carats. Cullinan I is the largest gem quality colorless cut diamond and is set in the head of sceptre of the British Crown Jewels. The second biggest diamond found, and 4th biggest cut diamond is 317.4 carat Cullinan II (Lesser Star of Africa), also part of British Crown Jewels.

The largest cut diamond is the Golden Jubilee with 545.67 carats. This diamond is brown and came from the Premier mine in 1985. The 273.85 carat Centenary Diamond, found in the Pretoria mine in 1986 and cut by DeBeers, holds the record as the largest flawless colorless diamond.

Right: Huge pink diamond Daria-i-Noor is set into the Iranian Crown Jewels. Photo: Siroos777
Of the fancy diamonds, the biggest vivid-yellow fancy diamond is 110.03 carat Cora Sun-drop Diamond found in South Africa in 2010. The largest pink diamond is 182 carat Daria-i-Noor from India, now in Iranian Crown Jewels. The French Blue (Tavernier Blue) was 69 carat blue diamond made for the French crown but lost in French Revolution and very likely later re-cut into the Hope Diamond (45.52 carats). The legendary Great Mogul weighed 280 carats and came from Golconda in India around 1650. It was probably re-cut as Orlov (190 carats), which is the part of the Tsar sceptre in the Kremlin Diamond Fund. The famous 105.60 carat Koh-i-Noor from India was mined by by the Kakatiya dynasty and served as the eye of the Hindu goddess. The diamond was stolen several times during its turbulent history, later was forcefully gifted to Queen Victoria and now is the part of The Royal Crown.

Diamond Names and Varietes
Fancy diamonds: usually clear with minor inclusions and impurities. The color can vary from common deep yellow or brown to less common light to saturated pink, red, orange, green, blue or violet.
Bort: the lowest quality small diamonds, often full of inclusions and impurites and display very poor clarity. These diamonds are often used as abrasives.
Carbonado: dark grey or black polycrystalline diamonds coming from central Africa or Brazil. Carbonados are found in alluvial sediments and often contain graphite and amorphous carbon which cause their dark color.
Diamond Occurence and Deposits
The worlds best and biggest diamonds came from the Premier Mine (Cullinan mine) in Pretoria, South Africa. Other famous mines include the Kimberley (The Big Hole) and the Finsch Mine. Other African mines are located in Namibia, Sierra Leone and Democratic Republic of Congo. The largest diamond volume producer in the world is the Argyle mine in Australia.

The Crater of Diamonds State Park in Arkansas, USA, is the only publically accessible diamond mine in the world. The only commercially operated diamond mine in the USA is the Kelsey Lake in Colorado. In 1990's in Lac de Gras in the Northwest Territories, Canada new diamond deposits were discovered - with the Ekati and Diavik Diamond mines being the most important. Bahia and Diamantina in Brazil are famous producers of diamonds, including the Sergio - the biggest diamond ever found.

South Indian localities were for millenia the only source of diamonds, however they are quite depleted now. Chagma Mine, Yimeng Mountains, Shangdong Prefecture in China is the biggest chinese diamond mine. Russia has huge diamond deposits in Sakha Republic (Yakutia) with huge pipes Mirny, Udachnaya and Internationalnaya.





Comments