Anatase - Mineral Properties, Photos and Occurence
Anatase is rare and highly aesthetic form of titanium oxide, which occurs almost exclusively in Alpine environment. It's great combinations with adularia and quartz are very popular among collectors. But it also has some unique physical properties, which are used in hi-tech applications.
Anatase is one of three most common mineral forms of titanium dioxide (TiO2), the other two being brookite and rutile. Anatase and rutile are similarly abundant, but easily distinguishable on the basis of habit (anatase displays steeper pyramidal faces), hardness, density and luster (anatase is softer, lighter and more metallic). Although anatase has been chiefly used as one of several white pigments, its unusual reactivity to UV light and water are leading to newly-imagined applications.

Anatase crystal on white adularia from Kharan District, Balochistan, Pakistan.
Size: 3 x 3 cm, Anton Watzl collection. Photo: Albert Russ
Crystal Structure of Anatase
Anatase crystallizes in the tetragonal crystal system. It typically forms dipyramidal or tabular crystals, with two growth habits. The most common form is acute-angle double pyramid, some of these being extremely elongated. The less common shape include flattened pyramid, or prismatic shapes. Oblique striations are often present, and sometimes the crystals show unusual accordion-like growths.
Anatase may contain Fe, Sn, V and Nb impurities but typical Alpine vein anatase is usually very pure.
 Left: Huge dipyramidal crystal of anatase from Hardangervidda, Norway. Size: 4 x 1.5 cm, Fossheim Steinsenter Museum collection. Photo: Zbyněk Buřival
Right: Very unusual complex crystal of anatase from Alp Lercheltini, Binntal, Switzerland.Size: 3 x 2.5 cm, Natural History Museum London collection. Photo: Zbyněk Buřival
Left: Huge dipyramidal crystal of anatase from Hardangervidda, Norway. Size: 4 x 1.5 cm, Fossheim Steinsenter Museum collection. Photo: Zbyněk Buřival
Right: Very unusual complex crystal of anatase from Alp Lercheltini, Binntal, Switzerland.Size: 3 x 2.5 cm, Natural History Museum London collection. Photo: Zbyněk Buřival
Anatase Physical Properties
Anatase is commonly black, but may occur as reddish to yellowish brown, dark blue, colorless or even white. Red, green and gray are rare. Occasionally, anatase will display splotchy lighter and darker patches. It is transparent when light-colored, but may appear opaque in dark specimens due to internal reflection. It displays weak pleochroism on 3 axes, but more strongly in deeply-colored specimens.
Cleavage is perfect on {001} and {011}. Twinning is rare on {112}. Anatase is brittle, with a subconchoidal fracture. Its luster is adamantine to metallic; its hardness is 5.5-6.0; its streak is white to pale yellowish-white; and its density is 3.79-3.97 g/cm3.
Alternate Names
Octahedrite is an earlier term for anatase, owing to the common octahedral-like habit of the crystal - in fact it is tetragonal dipyramid. Oisanite and dauphinite are obsolete place-names of principal occurrences in France. Wiserine is a name formerly uniquely applied to the honey-yellow-habit variety described above.
 Nice clear quartz cluster covered by dozens of anatase crystals from Hardangervidda, Norway. Size: 12 x 12 cm, Crystal Classics collection. Photo: Albert Russ
Nice clear quartz cluster covered by dozens of anatase crystals from Hardangervidda, Norway. Size: 12 x 12 cm, Crystal Classics collection. Photo: Albert Russ
TiO2 Polymorphs and Solid Solutions
Titanium di-oxide has 5 recognized or pending polymorphs:
Rutile, a tetragonal polymorph with a shorter vertical axis. Named by Abraham Werner, 1800; type locality Horcajuelo de la Sierra, Madrid, Spain.
Brookite, an orthorhombic polymorph named in 1825 by Serve-Dieu Abailard "Armand" Lévy after Henry James Brooke, amateur English crystallographer; type locality Prenteg, Gwynedd, Wales, UK.
Riesite, another monoclinic high-pressure polymorph derived from meteorite impact at Nordlinger Ries Crater, Swabia, Bavaria, Germany.
Akaogiite, a monoclinic high-pressure polymorph derived from meteorite impact, named after Masaki Akaogi, Professor at the Department of Chemistry, Gakushuin University in Tokyo, Japan.
TiO2 II, third high-pressure polymorph identified at Nordlinger Ries Crater, Swabia, Bavaria, Germany; awaiting IMA approval.
UM1991-08-O:Ti, a monoclinic polymorph forming tiny lamellae within Alpine anatase crystals.
Anatase itself is stable at room temperature, where it can form pseudomorphs after rutile. At high temperatures (between 550 and 1000 oC), anatase will recrystallize as rutile.
 Excellent striated anatase crystals from Balochistan, Pakistan. FOV: 3 x 2 cm. Collection and photo: Petr Fuchs
Excellent striated anatase crystals from Balochistan, Pakistan. FOV: 3 x 2 cm. Collection and photo: Petr Fuchs
Naming and Discovery
René Just Haüy, a French priest ordained in 1770, became fascinated with mineralogy shortly after his investiture. Haüy became a pioneer in the new field of crystallography after noting the perfectly smooth planes of fractured mineral specimens. His experiments with repeatedly cutting and breaking mineral samples led him to conclude that every crystal possessed a primitive nucleus or, as he termed it, an integrant molecule which could not be subdivided without destroying the fundamental identity of the crystal. He also recognized the power of the orderly arrangements of successive layers in creating crystal geometry. He published numerous reports on his investigations of mineral structures between 1784 and 1822. In these, he explored the idea of primitive crystal forms, and developed a mathematical theory to explain the appearance of crystals. His work on crude samples in the early 19th century ultimately laid the foundation for the 1902 X-ray discovery of microscopic crystal lattices.
Along the way, he named anatase in 1801 on the basis of the Greek anatasis (extension), referring to the vertical axis of anatase being longer than that of the similar rutile.
 Interesting combination of yellow anatase with yellow needles of rutile from Gasteiner Tal, Austria. FOV: 5 x 4 mm. Collection and photo: Petr Fuchs
Interesting combination of yellow anatase with yellow needles of rutile from Gasteiner Tal, Austria. FOV: 5 x 4 mm. Collection and photo: Petr Fuchs
Origin
Anatase is most commonly found in both hard rock environments, forming crystals in crevices of various schists, phyllites and gneisses, and smaller aggregates disseminated as a common detrital mineral in sediments. Low-temperature quartz veins in Alpine environments in central Europe are the best-known examples.
 Cluster of anatase crystals from Balochistan, Pakistan. FOV: 12 x 10 mm. Photo: Vítězslav Snášel
Cluster of anatase crystals from Balochistan, Pakistan. FOV: 12 x 10 mm. Photo: Vítězslav Snášel
Alpine vein anatase is typical for rocks with elevated Ti content - like phyllites, mica-schists, and gneisses. It is often associated with quartz, adularia, or less often with titanite or rutile.
Much less often, anatase can be present as a late hydrothermal phase in some granites or pegmatites. It can also occur in some carbonatite deposits.
Applications
Anatase, along with rutile, is the most commonly used form of TiO2 to create white pigments for use in paints, plastics, paper, ink, medicine, toothpaste, and even as a brightener in skim milk. Annual consumption in this form is about 4 million tons. However, industrial anatase is created in chemical facilities - titanium is recovered from ilmenite and rutile. Natural anatase is too rare and does not form deposits.
However, anatase has peculiar characteristics that affect the types of niche applications available, all dependent on its surface properties. It turns out that the surfaces of anatase particles hydrolyze, that is, they generate hydroxyl radicals (OH--) which are potentially carcinogenic or toxic to organic molecules. This leads to 4 different outcomes:
Manufacturers of sunscreen can take advantage of the whitening and UV-blocking capabilities of anatase to make the product, provided they first coat the particles with silica or alumina to prevent the particles from coming into contact with wet skin or water.
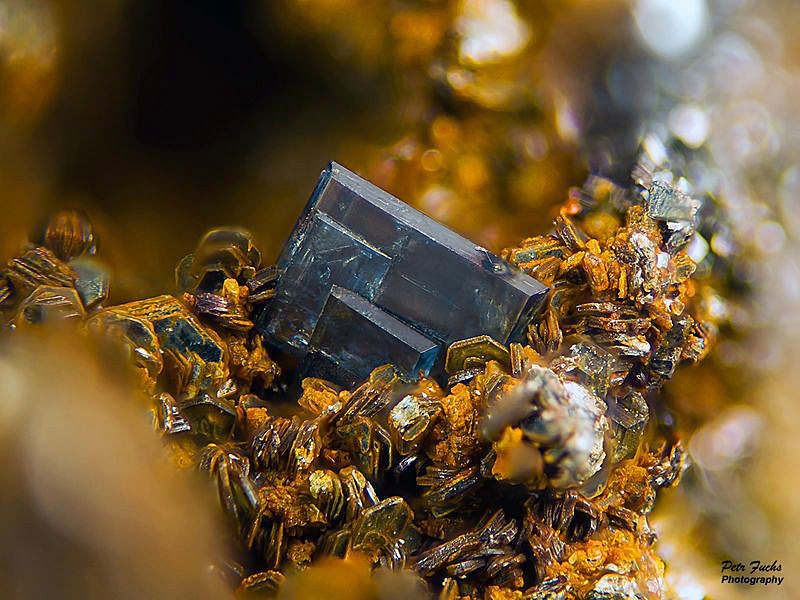 Unusual tabular blue anatase crystal from Krupka, Czech Republic. FOV: 2 x 1 mm. Collection and photo: Petr Fuchs
Unusual tabular blue anatase crystal from Krupka, Czech Republic. FOV: 2 x 1 mm. Collection and photo: Petr Fuchs
Manufacturers of cement and tiles can take advantage of the hydrolysis effect to create sterilizing and anti-fouling properties in the product; in the presence of water, the hydrolysis radicals will convert organic molecules to CO2 + H2O, and destroy micro-organisms.
Manufacturers of glass can take advantage of the hydrolysis to create a self-cleaning product, first introduced in 2001. Anatase powder is combined with an organic complexing agent to form a soluble mixture which can be spread evenly as a thin layer on a glass surface. Subsequent heating drives off the organic complex. The resulting glass will react to UV light photochemically, create hydroxyl radicals when wetted, and wash the dirt off in a sheet of water.
Manufacturers of solar panels can take advantage of the UV response of anatase to construct multilayer glass plates (called Grätzel cells) to generate currents from the electrons contained in dyes bonded to the anatase. This is a new technology which has yet to scale up for mass production. The principal obstacle to implementation appears to be the uncontrolled freezing or vaporization of the working fluid.
Occurrence of Anatase
The type locality is St. Christophe, Bourg-d'Oisans, Isère, France. There are numerous Alpine veins at many sites throughout the Swiss, French, Italian and Austrian Alps. In Switzerland, large crystals from Binn, Valais, and at Cavradi, Tavetsch, and Graubunden. In Norway, at Kragerø; fine crystals from Hardangervidda, Ullensvang; Slidre, Valdres; and Gudbrandsdalen. In England, from the Virtuous Lady Mine, Devonshire. In Wales, from Fron Oleu, near Tremadog, Gwynedd.
 Perfect anatase crystal on adularia from Kharan district, Balochistan, Pakistan. Crystal size: 1.5 cm, Anton Watzl collection. Photo: Albert Russ
Perfect anatase crystal on adularia from Kharan district, Balochistan, Pakistan. Crystal size: 1.5 cm, Anton Watzl collection. Photo: Albert Russ
Excellent anatase crystals come from Kharan district, Balochistan, Pakistan. In Russia, from the Lapcha Mine and Dodo Mine in Tyumen Oblast.
In the USA, at many localities: in Burke Co., North Carolina; Buckingham Co., Virginia; from Quincy, Norfolk Co., Massachusetts; at Placerville, Eldorado Co., California. In Canada, at Sherbrooke Township, Nova Scotia, and in Henvey Township, Ontario. In Brazil, at several carbonatite deposits, aggregating 500 million tons, in Minas Gerais and Par ́a.





Comments