Calcite - Mineral Properties, Photos and Occurence
Of all sedimentary minerals, calcite is probably the most widespread, contributing a full 2% to the volume of the Earth's crust. It occurs in endless crystal forms with many different colors. With such variety it is not surprising that some collectors specialize on calcite alone.
Calcite Structure
Calcite is calcium carbonate (CaCO3) and belongs to trigonal (rhombohedral) crystal system. It occurs in a wide variety of crystal habits - rhombohedrons, scalenohedrons, tabular and prismatic crystals and just about everything in between. Calcite also exhibits several twinning types. Such variability results in several thousands known crystal forms of calcite!
Other polymorphs of calcium carbonate are hexagonal aragonite and vaterite, which are metastable and recrystallize into calcite under Earth's near-surface conditions. However, this process can take many million years to finish.

Calcite's structure easily accommodates significant amounts of Mg, Fe, Ba, Sr, as well as smaller amounts of Co, Mn, Pb, Zn and many other elements. Elevated content of Co causes the pink coloration; structural defects are probably the reason for the blue.

Physical Properties of Calcite
Pure calcite is transparent and colorless but also occurs in white, gray, yellow, orange, red, brown, black, or rarely, also blue. Calcite defines the hardness 3 on Moh's scale with specific gravity 2.71 g/cm3. It has vitreous luster and perfect cleavage in three dimensions. If you strike a piece of calcite with a hammer, it will split into many small rhombohedrons along its cleavage planes.

Calcite readily dissolves in diluted hydrochloric acid or vinegar. It is also slightly soluble in water, which causes dissolution and reprecipitation of calcite along fissures in limestone rocks. These processes lead to the formation of karst landscape with famous caves and dripstones. Similar processes cause the corrosion of concrete.

The pure transparent calcite variety with clearly visible birefringence (double refraction) is called Iceland spar. The birefringence effect was first described on calcite by a Danish scientist Rasmus Bartholin in 1669. The Iceland spar calcite is used in some special optical systems. Calcite often shows phosphorescence and fluorescence: some even display fluorescence in both short and long wave UV.

Origin of Calcite
Calcite often forms crystals easily reaching 20-30 cm inside low temperature hydrothermal veins occurring within limestones. Some such sites have evens produced significant amounts of crystals over 0.5 m in size. Medium temperature hydrothermal veins containing Pb-Zn-Ag minerals or fluorite and baryte also usually contain abundant calcite crystals, sometimes accompanied by less common rhodochrosite, dolomite or ankerite.
Many alpine veins in calcium rich rocks produced nice calcite crystals or Ca-rich zeolites. Calcite is also widespread in veins and bubbles (geodes) inside Ca-rich volcanic rocks. Many marine organisms use calcite to build their shells, especially molluscs and certain micro-organisms. Billions of such shells sedimented on the ancient sea floors to form thick limestone strata.

Calcite as a Rock Forming Mineral
Calcite is very important rock forming mineral. Many marine sediments - like marlites, schists, opoka and others - contain significant amounts of calcite. Limestone is made of almost pure calcite. When these sediments are buried during subduction into the Earths crust or get into the contact with hot magma, they are metamorphosed into marbles or calc-silicate rocks. Sometimes the sediments are buried so deep that they melt into carbonate magma and create carbonatite rocks. Calcite can be also remobilised by fluids and redeposited into metasomatic rocks like skarns.

Industrial Use of Calcite
Limestone is a critical resource for the construction industry. Both cement and lime are produced by heat decomposition of limestone/calcite. Without calcite, no modern building could exist. Good quality marble is often used as a construction or decorative material. Calcite is also very important resource of calcium for chemical industry and it is used in paper production, smelting, food industry, glass production and ceramics.
Crushed limestone is also used to balance pH of acidic soils or acid mine drainange (highly acidic waste waters). Calcium hydroxide is very important to remove sulfur dioxide from the smoke of the coal power plants. Gemmy flawless Iceland spar calcite is used in optics.

Occurence of Calcite
Calcite occurs all over the world. Honey-colored scalenohedrons can be found in Malmberget, Sweden. Excellent crystals up to 0.5 m large have been found in Štramberk and Prachovice, Czech Republic. Many hydrothermal vein deposits produce nice crystals - especially Příbram in Czech Republic, classic sites such as Alston Moore, Egremont, Frizington and Weardale in England and many old deposits in Romania, Bulgaria, Slovakia or Germany. Famous bicolored calcite balls were found in Herja, Romania. Crystals measuring up to 7 m large from Helgustadir on Iceland are probably the biggest in the world.

Elmwood Mine in Tennessee is famous for its big scalenohedrons. Joplin, Missouri has yielded crystals up to 1 m in size. Other sites in USA include many fluorite deposits in Illinois and Missouri and hydrothermal veins in Colorado and Iowa. Guanajuato, Mina Ojuela and many other sites in Mexico produced great calcite clusters. Nice crystals have also been found in Quebec, Canada or in Huanzala, Peru.

Nice crystals from African hydrothermal veins come from Katanga in Congo, Tsumeb in Namibia and El Hammam in Morocco. Exceptional optical calcite crystals up to 30 cm large have been found in Dalnegorsk in Russia. Many excellent calcites have recently been discovered in China, especially in Hubei, Hunan and Inner Mongolia provinces. Excellent calcites accompanied by zeolites occur in volcanic geodes in India.

Calcite Varietes
Although calcite occurs in many forms and colors, it has surprisingly few varieties. Cobaltoan and manganoan calcites are chemically different while other types and varietes differ only in shape, color or their origin.
Cobaltoan calcite (cobaltocalcite)
Cobaltocalcite is very popular among collectors because of its attractive appearance. It has pink, light purple, pinkish brown or pinkish gray color but the most popular specimens are a deep vivid pink color. It was originally described from the Calamita mine on Elba island, Italy. Very nice pink scalenohedrons have been found in Kolwezi, Congo. The most famous locality is probably Bou Azzer in Morocco, which still produces excellent quality , brighty colored cobaltocalcite clusters. Localities of this uncommon mineral include Slovakia, Germany, Switzerland, UK, Australia, Mexico and USA.

Manganoan Calcite (Manganocalcite)
Unlike cobaltocalcite, managnoan calcite often has white, yellowish or light gray color and it is difficult to distinguish from common calcite. It was originally described from Banska Stiavnica in Slovakia. Manganocalcite is widespread especially at hydrothermal deposits with other manganese minerals. Many localities for manganocalcite are in Slovakia, Bulgaria, Romania, China, Russia, USA and Peru. The manganocalcite is probably much more widespread but unless properly analysed, it appears to be normal calcite.

Agaric Mineral
The soft and chalky calcite formed by the pricipitation in caves or fissures is called agaric mineral or rock milk. It is easily dissolved or physically damaged, but slowly recrystallizes into flowstone over time.
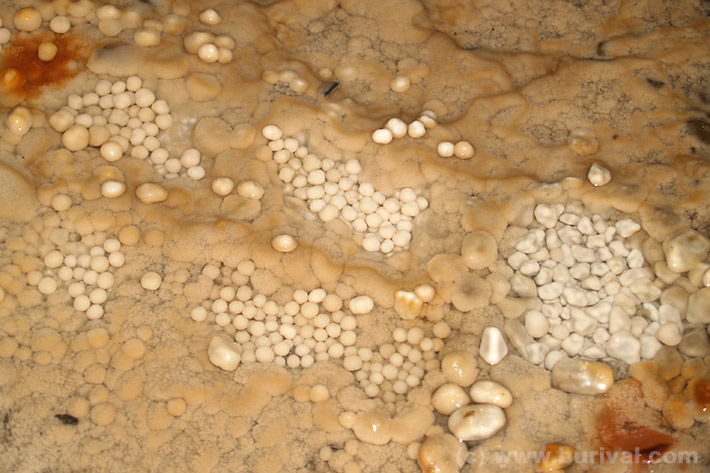
Flowstone
Almost all solid karst features - such as stalactites, stalagmites, cave pearls and others - are formed by flowstone. This type of calcite precipitates from karst waters draining the fissures and caves inside the limestone (or much more rarely marble) bodies. Flowstone precipitates very slowly and formation of karst caves with stalactites and stalagmites requires millions of years.

Dogtooth Calcite
Groups of typical scalenohedron crystal groups are called dogtooth calcite because the whole vein cross-sections with scalenohedrons pointing inside the vein from both sides look like dogs' teeth. This type is typical for hydrothermal veins and pockets in carbonate rocks. Typical colors include white and yellow, and less commonly brown and red.

Nail Head Spar
Calcite crystals with short or long pseudohexagonal prism and typical traingular faces at the top is called nail head spar. This type of calcite is very widespread on many hydrothermal veins and can be of many colors and sizes.

Iceland Spar (optical calcite)
Excellent optical quality calcite with nicely visible birefringence is called Iceland spar. The first crystals of this quality were found on Iceland but their occur on many other sites worldwide.






Comments