Malachite - the Copper Green
Brilliant green color makes malachite a very popular and widely-recognized stone. Bright color, aesthetic specimens and reasonable price make this interesting mineral very popular among collectors.
Structure od Malachite
The crystal system of malachite is monoclinic in nature, which means that it features three axes that are not equal in length to each other. Two of these axes stand at right angles to each other, and the third may be at any other angle aside from ninety degrees. Malachite's chemical formula is Cu2CO3(OH)2.
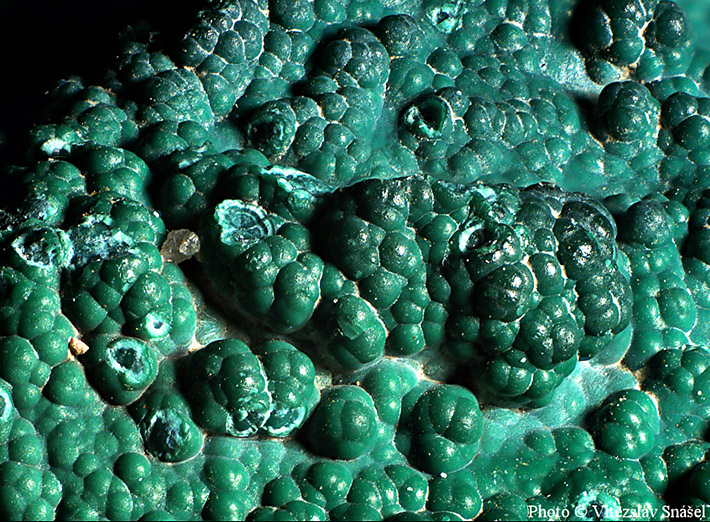
Commonly, malachite forms as microscopic crystalline crusts, or as long, splintery pieces. It is difficult to find individual crystals that are not tiny. When larger crystals do exist, they are usually prismatic, or acicular. Malachite often forms botryoidal masses, banded masses, as a crust on top of other minerals, or as stalactites.
Malachite often replaces azurite, resulting in highly sought-after pseudomorphs. Sometimes, an incomplete replacement results in an azurite-malachite pseudomorph.

Physical Properties of Malachite
Unlike many other minerals, malachite has only one color: green. It may occur in any shade from light to dark green, and it is often banded with various different shades. In some instances, it may be sparkling; it is an opaque mineral, but the very thin splintered pieces may appear translucent.

Malachite is quite soft, registering at a 3.5 to 4 on the Mohs scale of hardness. When fractured, it splinters; it features a basal cleavage, but this is rarely noticeable as it is most common in the microscopic crystal specimens. The luster of malachite may be silky, dull, or vitreous, depending on the particular specimen. As a carbonate, malachite is easily decomposed by any acid.
The beautiful color and relative softness of malachite make it a commonly used ornamental stone for carvings and figures. It is also sometimes used in cabochons and beads, particularly in pendants and necklaces. Huge malachite deposits in Russia resulted in large vases or even baths made of the layered malachite. However, its softness and very poor chemical resistance make these items useless for anything other than ornamentation. Malachite was also used as a pigment.

Similar Minerals
Needle like crystals of malachite are very similar to brochantite, which is a copper sulfate and may be quite widespread at some copper deposits. Brochantite is usually more dark green and does not fizz in acids.
Botryoidal malachite is extremely similar to pseudomalachite, which is a copper phosphate that is common in some copper deposits. Pseudomalachite forms botryoidal aggregates of very similar color and shape as malachite, and it often has the same internal layered structure. Unlike malachite, pseudomalachite does not fizz in acids.

Secondary copper minerals are usually green and very often occur in mixtures. Even a couple dozen various copper minerals are not uncommon at one location. Malachite and azurite are the most common and can be easily identified by fizzing in diluted hydrochloric (muriatic) acid. Of course, it is best not to test this on your favorite crystal specimens at home!
Origin
Malachite is a typical secondary mineral. It originates by decomposition of primary copper minerals - usually copper sulfides like chalcopyrite, tetrahedrite, bornite, copper oxide cuprite or native copper. Sulfides are minerals which originate in the reducing environment without oxygen. When erosion reaches the level of the sulfide deposit, the surface water, oxygen, CO2 and other substances interact with sulfides and cause their oxidation and decomposition into secondary minerals. Copper sulfides decompose into a wide variety of green and blue carbonates, sulfates, phosphates or even silicates. Malachite is a typical and very widespread product of such weathering of copper deposits.

Occurrence of Malachite
Originally, beautiful malachite specimens came from Yekaterinburg Oblast in Russia's Ural Mountains. However, today, malachite is found in several other places. The Katanga mines in the African Congo are well known for their uniquely-shaped malachite. This location is also one of the best for beautiful sparkling malachite, as well as banded and concentric specimens, and malachite stalactites. Another African source of malachite is the Emke Mine in Namibia.

Touissit and Kerrouchene in Morocco produce beautiful malachite, as well. In Australia, Burra Burra, the Rum Jungle, and the Sir Dominick Mine are well-known malachite locations. More recently, China has entered the scene, providing amazing specimens and stalactites. Malachite pseudomorphs can be found in France, and large deposits exist in Bahia, Brazil, and at the Milpillas Mine in Mexico. The mineral may also be found in Arizona, at several different mines.





Comments