Cinnabar - Epic Mineral Overview
Cinnabar is bright red mercury sulfide, which is a main ore of mercury and used to be a source of red pigments for milleniums. Cinnabar (and mercury) had been a key to mining precious metals until beginning of the 20th century and also saved a millions of teeth.
Cinnabar is a brick-red, bright scarlet variety of simple mercury sulfide (HgS) that is the most common source of elemental mercury. Its common occurrence as low-temperature (~100 °C) hot-spring coatings and fracture-fillings in the near-surface environment allowed cultures throughout the world, beginning in the Neolithic, to use the powdered mineral as a convenient pigment for ornamentation, known variously as vermilion or Chinese Red in painted decorative objects.
Crystal Structure of Cinnabar
Cinnabar is a member of the trigonal crystal system, characterized by tabular to rhombohedral crystals, or as encrustations or granular or massive forms. Individual crystals may show striations. Cinnabar crystals are often twinned, usually as contact twins. Unlike most minerals, the internal structure of cinnabar is built of neverending strings of S-Hg-S-Hg, which form spiral. Both left and right oriented spirals exist.
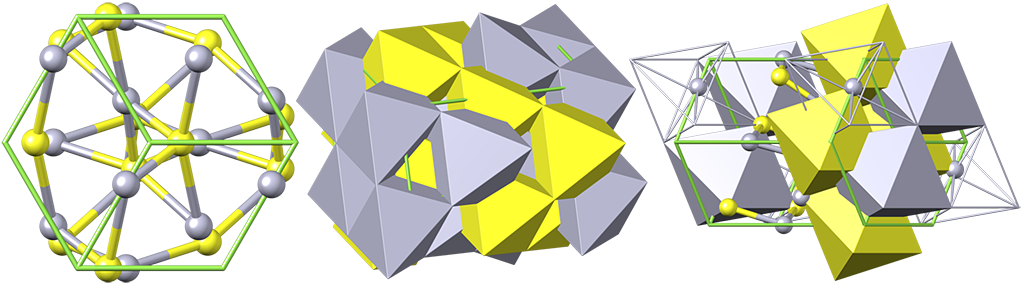
Cinnabar structure can accommodate smaller amounts of Se and Te instead of sulfur. Cinnabar is typical for low temperature environments and it is estimated that it mostly forms about 100 °C. The cinnabar structure changes to cubic metacinnabar above 344 °C. However, some cinnabar is obviously result of recrystalization of metacinnabar but there is no evidence for reverse process (in nature). The stability limit temperature of metacinnabar can drop as low as 240 °C in the presence of Fe and/or Zn in its structure.
Physical Properties of Cinnabar
Cinnabar is typically a bright or brownish red but may be degraded to dull, lead-grey tones. The streak is red-brown to scarlet. Pure cinnabar is transparent in thin pieces. The powdered cinnabar has been used as a pigment since ancient times due to its vivid color.

Cleavage is perfect on {1010} in prismatic specimens. Twinning, if present, is penetrative. Luster ranges from adamantine to dull. Dull specimens are typically full of impurities, whereas pure specimens will be bright red. Fracture is uneven to subconchoidal. Its hardness ranges from 2.0-2.5; and its density is quite restricted, between 8.000-8.176. By weight, cinnabar is 86.22 % Hg and 13.78 % S.
Varietal Names
Hepatic cinnabar or paragite is a brown-black variety, considered impure, described from the Idrija Mine complex in Slovenia.

Polymorphs
Three polymorphs (minerals with the same formula but different structure and physical appearance) are recognized:
- cinnabar - most common low temperature trigonal form,
- metacinnabar - a low-temperature cubic form discovered in 1870 at the Redington Mine, Napa County, California,
- hypercinnabar - a high-temperature hexagonal form.
Similar Minerals
Massive forms of red hematite Fe2O3can be very similar to massive cinnabar, but its specific gravity is only 5.26 (cinnabar is above 8). However, distinguishing these two in mixed aggregates might be quite tricky. What is even worse, they can occur together. Also massive red cuprite Cu2O or minium Pb3O4 look quite similar, have high specific gravity and red streak. But both are quite rare and do not occur on mercury deposits.

Associated Minerals
The most common associated minerals are those expected in low-temperature hydrothermal systems: baryte, dolomite, ankerite, siderite, quartz and pyrite. Less common are realgar, stibnite, marcasite or calcite. Very often is present liquid native mercury, which originates by decomposition of cinnabar.
In addition, there is an unusual occurrence of myrickite, a mineral aggregate (described from Myrick Spring, San Bernardino, California) consisting of agate, chalcedony or opal containing blebs of cinnabar.

Naming and Discovery
Cinnabar seemingly has a multiplicity of name origins. In Greek, it was known as kinnabari; in Sanskrit as sugara, in Persia, as shangarf, later Arabicized to zinjifrah (‘dragon’s blood’); in Latin, as both minium (‘red cinnamon’) or cinnabaris. Its discovery and use predate historical records, so there is no credited ‘discoverer.’ Beware that minium is currently name of an IMA valid mineral - brick-red mercury oxide Pb3O4!
Origin
The typical mode of formation of cinnabar is as a vein-filling or fracture coating in near-surface volcanic systems or in alkaline hot springs. It is very typical low-temperature hydrothermal mineral, quite often associated with minerals of Sb and As. It can also form impregnations in certain limestones or bitumenous shales.

Applications
Mining of cinnabar originated at least as far back as the Neolithic (7,000 BC). It is self-evident that the earliest use of cinnabar was as a crushed and powdered pigment. It was identifiably used in the Near East as a rouge cosmetic; in the New World in the Olmec culture (1500 BC-400 BC) as ornamental powder, and in China in the Song Dynasty (960-1279 AD). The Chinese perfected the art of combining the powder with a lacquer, creating the widely recognized shade, ‘Chinese Red.’ Chinese carved lacquer (Qidiao) was often colored with cinnabar pigment.
The cinnabar is often on the top of the nonsense "top toxic minerals" lists. However, pure mercury sulfide itself is highly inert even if consumed. The "toxicity" of cinnabar is caused by two main factors: 1) release of elemental mercury when heated and/or pressed; 2) inclusions or impurities of other toxic minerals, especially metallic native mercury. The mercury toxicity is extensively discussed in the native mercury overview.

Elemental mercury vapor is easily liberated from cinnabar with mild heating. It is a fast-acting neurological toxin with profound consequences for human exposure (headache, muscle twitching, weakness, loss of sensation and death). Symptoms of mercury poisoning were recognized as long ago as the Roman era, and the use of cinnabar as a decorative or jewelry-related item has been drastically curtailed ever since.
Uncontrolled dispersion of liquid mercury and mercury vapor from industrial sites and metallurgical refining have added an unanticipated biologic burden to the environment in the form of methyl mercury (CH3Hg), which remains a latent and invisible threat to the aquatic portion of the food chain.

Elemental mercury is easily recovered from cinnabar by mild heating. A typical recovery scenario at an operating cinnabar mine would involve setting up a series of inclined empty cylinders (kilns). Each kiln was open at each end, heated from below, and rotated slowly by a pinion gear engaging a ring gear on the outside of the kiln. As the heated kiln slowly rotated, coarse cinnabar would be dumped in the upper end of the open kiln and gradually travel down the length of the kiln, constantly giving off mercury vapor to be captured by piping installed above and diverted to a collection facility. Such operations were extremely messy, resulting in thousands of globules of liquid mercury droplets scattered all over the adjacent ground downwind of the collection area.

Elemental mercury is a unique element, and in its heyday possessed several valuable industrial properties, namely its liquidity and conductivity at room temperature, its physical flexibility; its toxicity, and its combinatorial capacity. One favored use was as a gravity switch in electrical applications. Thermometers and barometers made wide use of mercury because of its thermal stability. Its toxicity was beneficial when used as a fungicide to protect seed crops and as a pesticide to combat worms. Combinatorially, for decades mercury was mixed with other metals to make dental fillings. In precious metals mining in the 1800s, mercury was mixed with the pulverized rock generated by stamp mills to recover the valuable gold and silver ore.
Today, legacy mercury use continues in some batteries and light bulbs, but its use is declining because disposal regulations are designed to limit human exposure. Industrial practice today is to find any reasonable substitute for mercury. The chemical industry does continue to consume large amounts of mercury to manufacture chlorine and caustic soda (sodium hydroxide) during electrolysis of brine.

The major environmental issue is also artisanal mining. As the demand drives gold price up, the artisanal mining is blooming again. The cyanide leaching is not an option for small scale or artisanal miners, so the mercury demand is growing too. Liquid mercury is used for amalgamation recovery of gold, which is very effective but also very dangerous and producing huge environmental issues. Some estimate that artisanal (illegal) mining produces up to the 80% of the worldwide mercury pollution, lead by China and Mexico miners.
Occurrence
Two of the better-known occurrences of cinnabar are the Almaden Mine in Spain and the Idrija Mine in Slovenia.
Almadén, Ciudad Real Province (Spain). The Almaden Mine is located in south-central Spain and is credited with the largest amount of liquid mercury metal produced in the world, totaling about 250,000 metric tons of metal over a 2,000 year lifespan. The historical facts about the scale of Roman mining there are sketchy. Documentation from the Medieval Period shows that two German bankers administered the mine during the 16th and 17th centuries. The importance of cinnabar to the Spanish grew because of the New World innovation of amalgamation to extract metal from silver and gold ore.

Working conditions in the mine were sufficiently dangerous that willing workers would not remain on the site, and the bankers appealed to the Spanish crown to supply convict labor. In 1566, The King of Spain agreed to a trial set of 30 convicts and increased the contingent to 40 in 1583. Workers were well treated, but complaints about sickness from mercury poisoning (signaled by a 24% death rate) led to a full royal inquiry in 1593. North African slaves were added to the work gangs, and by 1613 were twice as numerous as the original convict allotment.
The state took over full control of the mines in 1645 and continued to use prisoners for labor. By the end of the 18th century, safer working conditions were in place, and free laborers were once again willing to work in the mines. The penal component of the workforce was shut down in 1801. Rothschild banking interests leased the mine from the Crown after first having acquired the Idrija Mine in Slovenia (see below). This gave the Rothschilds a true monopoly mad financial windfall on European mercury production. Spain took back control in 1863.

A new operating council formed in 1916 and instituted technology and safety improvements. The mine operated continuously with ramped-up production, and a maximum yearly output was attained in 1941, but price collapses over the next 30 years, due to falling demand, made the continued viability of the mine questionable, and the property ceased to operate in 2000. The mine still has an enormous reserve of unexploited metal.
The physical geology of the deposit is relatively simple. Two quartzite members of a Lower Silurian shale package, thoroughly impregnated with cinnabar, lie near a phreatic explosion breccia. The shales immediately below, within and above the two quartzites are not significantly altered, but the overlying volcanics are. The Ordovician black shales below are sulfidic and contain anomalous mercury. The geologic hypothesis is that two quartz-bearing sands lying on the seafloor were selectively and separately invaded before diagenesis by cinnabar-rich solutions, from the breccia zone, traversing the mercury-rich shales. The package was then folded and heated to low-grade metamorphism (210-270 °C). This in no way explains why there should be two distinct, identical episodes of mineralization separated widely in time.

Idrija Mine, (Slovenia). According to local legend, a bucket maker at a nearby spring saw globules of liquid mercury in 1490. The formal discovery of the first rich ore vein is celebrated as June 22, 1508. Over its lifetime, Idrija accounted for 13% of world mercury production, amounting to 147,700 tons of mercury. Two main shafts were completed, Francis’s Shaft in 1792, a large haulageway, and the Joseph Shaft, 420 m deep, connecting all 15 levels of the mine which includes 700 km of underground workings. Technologically, the mine was home to the largest (13.6 m diameter) water wheel in Europe, which pumped successfully for 160 years, Mine closure was initiated in 1986 and completed in 1995, with the site now a UNESCO World Heritage Site available for tourism.

Geologically, the Idrija deposit is characterized as exceedingly structurally complex. The published cross section, supported by 3 Ph.D. dissertations over a 30-year period, suggests a complicated extensional regime affecting Middle Triassic sediments, with the Idrija deposit fully contained within a graben about 400 m wide, in which an mineralized fault block is 100 m wide.
In China, mines in the Guizhou Province are reported to contain fine crystals at Tongrin, Wanshabchang; in Hunan Province, the Tsar Tien Mine is noted for exceptional twinned crystals.

California contains the namesake mines of New Almaden, Santa Clara County and New Idria, San Benito County, as well as the Mount Diablo Mine, Contra Costa County and the Hastings and St. John's Mines in Vallejo. The description of the devastating impact of the rotary kiln operation mentioned in Applications is based on personal observations I made while on a geologic visit to New Idria in 1980.
Terlingua, Brewster County, Texas is a world-famous mercury district. Cinnabar there occurs as replacements following a contact between Lower Cretaceous Devils River Limestone and the Upper Cretaceous Grayson Clay. Discovered in 1902, the Chisos Mine operated until 1943, producing 100,000 ‘flasks’ (76 pounds per flask, a U.S. Hg measure), from 23 miles of workings on 17 levels. The Mariposa Mine, 7 miles west, operated actively from 1895 to 1911, credited with 30,000 flasks. The Study Butte Mine, 5 miles east, operated from 1902 until WWII, producing cinnabar from fractures within a syenite intrusion.

In Peru, the Huancavelica area (450 km south of Lima) boasts a 3,500 year history of cinnabar mining. The site, known as the Mine of Death, was under the control of the Inca until the Spanish took it over in 1564. From that time forward, 36,000 tons of mercury were recovered until mine closures in 1974. Modern research using sediment cores from nearby lakes establish a record of mercury pollution as early as 1400 BC. Throughout the early years, the pollution burden was 3 to 5 times background until the rise of the Inca Empire ca, 1460 AD, at which time the pollution level attained 30x background. The apparent reason for the spike in values is the switch from grinding the ore for pigment to heating the ore for mercury recovery. The vapors laid down a wide, regionally extensive footprint of contamination. There is no direct evidence as to why the Inca wanted to recover liquid mercury, but speculation has it that it may have been used a polishing agent for gold and silver objects.
In Nevada, the Cahill mine, Poverty Peak District, Humboldt County and Antelope Springs District, Pershing County are the two principal cinnabar sites. Antelope Springs operated intermittently from 1912 to 1931. At its peak, it used one 3’ diameter, 40’ long rotating cylinder to produce 600 flasks per month. In Arkansas, Murfreesboro, Pike County was the site of a very short-lived Depression-era mining boom for mercury. One thousand people flooded in to work in 1931 for a decade before the ore was exhausted. In Alaska, the Red Devil Mine on the middle Kuskokwim River operated from 1921 until 1971 under a variety of operators.

In Mexico, historic cinnabar specimens grow on brilliant white quartz at Charcas, San Luis Potosi. In the Caribbean, Dominica, Lesser Antilles hosts cinnabar along the west coast, near the sulfur springs at the southern end of the island.
In Italy, Mount Amiata, Tuscany is the site of one of Italy’s largest lava domes that erupted 300,000 years ago and continues deposit cinnabar in a geothermal field near the town of Bagnore, southwest of the domes.
In Slovakia, the village of Rudňany discovered cinnabar in the 13th century. Iron-ore mining developed much later into a major enterprise. The whole hydrothermal vein swarm in the area hosts many places with typical siderite-cinnabar-tetrahedrite ores.
Other notable places are: In Serbia: Mount Avala; In Kazakhstan: Hydercahn, in the Fergana Basin; In Kyrgyzstan: Chauvai, Alai Range; In Ukraine: Nikitovka (Horlivka), Donets'ka Oblast; In the Philippines: Puerto Princesa; In Egypt: Giza.
Literature
- Covelli, S., Faganeli, J., Horvat, M., & Brambati, A. (2001). Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic sea). Applied Geochemistry, 16(5), 541-558.
- Dickson, F. W., & Tunell, G. (1959). The stability relations of cinnabar and metacinnabar. American Mineralogist: Journal of Earth and Planetary Materials, 44(5-6), 471-487.
- Gettens, R. J., Feller, R. L., & Chase, W. T. (1972). Vermilion and cinnabar. Studies in Conservation, 17(2), 45-69.
- Liu, J., Shi, J.Z., Yu, L.M., Goyer, R.A. and Waalkes, M.P., 2008. Mercury in traditional medicines: is cinnabar toxicologically similar to common mercurials?. Experimental biology and medicine, 233(7), pp.810-817.
- McCormack, J. K. (2000). The darkening of cinnabar in sunlight. Mineralium Deposita, 35(8), 796-798.
- Sun, S. R., & Dong, Y. H. (2005). First-principles study of the phase transition of HgS from cinnabar to rocksalt structure under high pressure. Physical Review B, 72(17), 174101.
- Takacs, L. (2000). Quicksilver from cinnabar: the first documented mechanochemical reaction?. JOM, 52(1), 12-13.
- Waples, J. S., Nagy, K. L., Aiken, G. R., & Ryan, J. N. (2005). Dissolution of cinnabar (HgS) in the presence of natural organic matter. Geochimica et Cosmochimica Acta, 69(6), 1575-1588.





Comments