Magnesite Mineral: Composition, Crystal Structure, and Geological Occurrence Explained
Magnesite is not the most popular mineral for collectors but it can form outstanding specimens and crystals. However, its principal use is as a magnesium ore and a special refractory - a material where resistance to extreme temperatures is fundamental to an industrial process.
Crystal Structure of Magnesite
Magnesite is a simple magnesium carbonate with the formula MgCO3 and belongs to calcite group minerals. Like all its carbonate relatives, it crystallizes in the trigonal system. Mineral specimens are ordinarily massive, rarely rhombohedral or as hexagonal prisms. It also occurs as thin prismatic needles, coxcombs, grainy masses, or in small rounded balls.
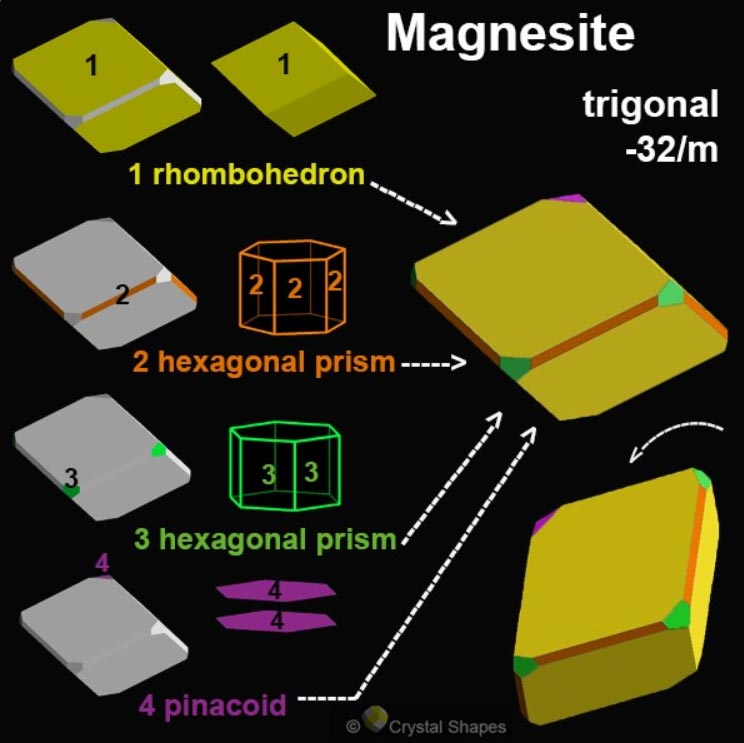
Magnesite has the same structure as other members of the calcite group. It forms complete solid solution with siderite FeCO3 and limited with calcite CaCO3 and rhodochrosite MnCO3. The miscibility with dolomite CaMg(CO3)2 is very limited because of their different structure, complete solid solution exists only at extremely high temperatures.
Physical Properties of Magnesite
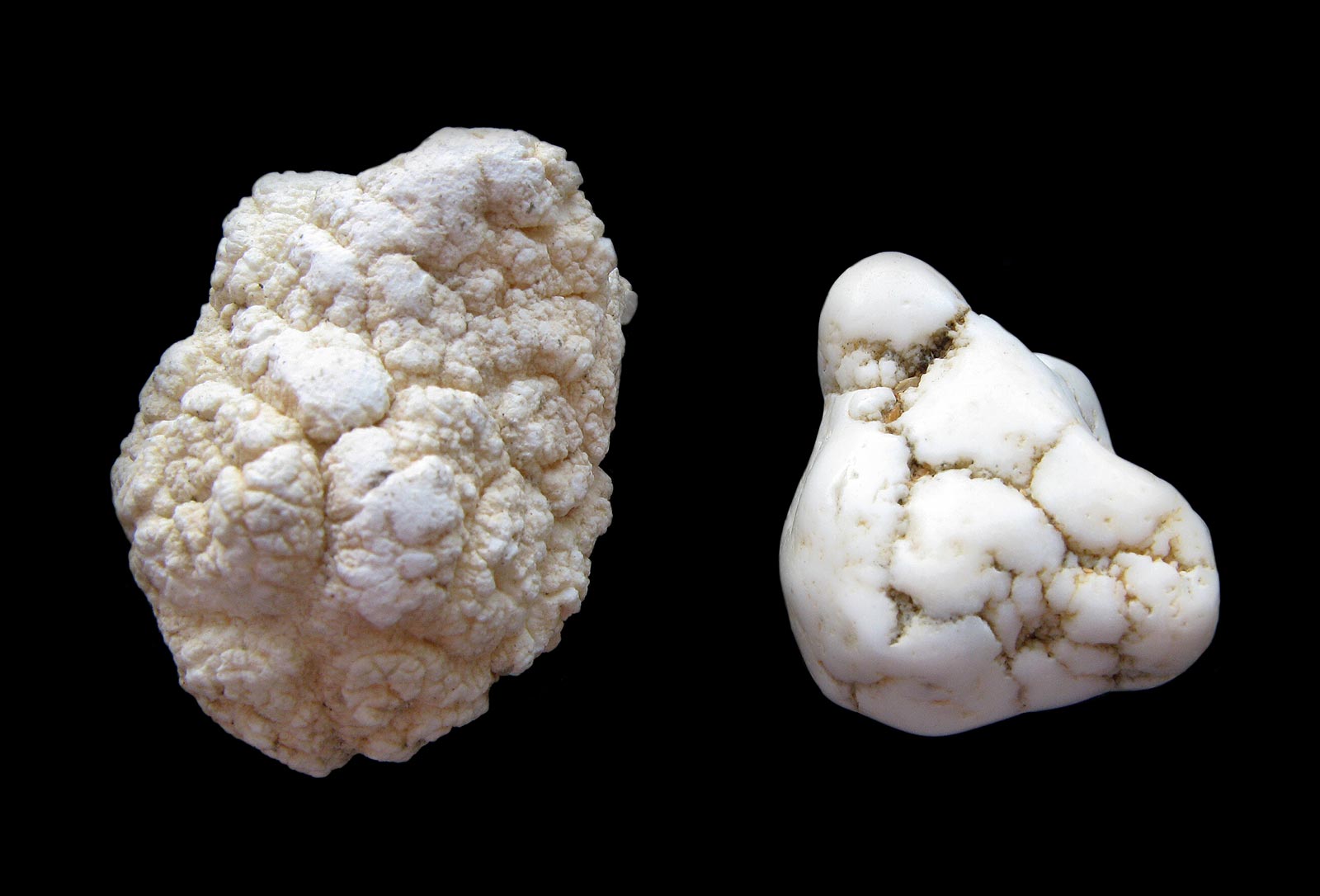
Magnesite is generally colorless to white, but may develop as yellow, pale brown, gray, faintly pink, or a lilac-rose. It often appears as a porous, dull, microcrystalline mass resembling unglazed porcelain. It may be a fluorescent or phosphorescent pale green to pale blue under UV, and also be triboluminescent. (Fluorescence is the production of unheated light by direct excitation; phosphorescence is the delayed production of unheated light after the cessation of excitation; triboluminescence is the generation of light by the rupture of chemical bonds).

Magnesite has perfect cleavage on {1011}. It is transparent to opaque. Its luster is variously described as vitreous, earthy or dull. Its fracture is conchoidal; the mineral is brittle. Its hardness is 3.5-4.5. Its streak is white and its density is 3.0-3.2.
Similar minerals
Magnesite is very similar to its carbonate relatives and often occurs together with calcite or dolomite. Massive fine grained magnesite nodules or veins are often impregnated by silica and sometimes hard to distinguish from chert and opal.
Associated minerals
Magnesite is generally found in association with antigorite, calcite, chlorite, dolomite, periclase, talc, brucite, artinite and wollastonite.

Naming and Discovery
Magnesite is simply named for its magnesium content in carbonate. There is apparently no documented or anecdotal information attesting to its original discovery.
Varietal names
Bitter spar is an informal name, likely derived from the property of sticking to the tongue when licked, owing to its inherent porosity.
Breunnerite or ferro-magnesite is a variety with significant amount of Fe2+ (siderite component), which is pale brown to brown.
Origin
On Earth, magnesite is characteristically found as a weathering product of magnesian rock types typical of oceanic mountain belts that contain ultramafic rocks such as eclogites, peridotites and ophiolite, and minerals such as serpentine, pyroxene, olivine and diopside. At the Earth’s surface, the magnesite will accumulate in the soil (regolith) overlying these rocks, and the proximate cause is the dissolution of magnesium-bearing minerals by carbon dioxide circulating in near-surface groundwaters.

At depth, removed from the weathering environment, magnesite can form from the metasomatic carbonation of eclogites, peridotites and similar ultramafics under the conditions of elevated temperatures and high pressures found in greenschist-facies metamorphism.
In marine sedimentary settings, magnesite formation is attributed to an association with dolomite in reefal environments subjected to temperature fluctuations above and below 40 °C (104 °F), suggesting that this boundary must be crossed repeatedly to permit dissolution and reprecipitation.

Beyond Earth, magnesite has been detected by analysis in at least one meteorite and by infra-red spectroscopy on Mars from orbiting satellites. The source and origin of this magnesite is unknown.
Applications
Magnesite (MgCO3) and its downstream product, periclase (MgO), are critical components of industrial processes that depend on materials capable of physical survival in the face of extreme temperatures. Magnesite is burned to produce periclase, which is then used to line incinerators, blast furnaces and kilns.
The burning treatment of magnesite/periclase is referred to as calcination, a thermal treatment which results in a ‘light burnt’ product usable between temperatures of 450 °C to an upper limit of 900 °C. In this temperature range, the product has a good surface area and good reactivity. Above 900 °C, the product is termed ‘dead burnt’ and loses its fine crystalline structure and degrades to a chemically inert material that is ideal for use in furnace linings.
Another widely used high-temperature use for magnesite is the manufacture of tiny cups (‘cupels’) required for completing fire assays of gold and silver.
In less extreme settings, magnesite is incorporated in flooring material as a binder, and is also employed as a filler or catalyst in the manufacture of synthetic rubber, and a variety of magnesium chemicals and magnesium-based fertilizers.
In jewelry-making, fine grained magnesite can be shaped into beads by cutting, drilling and polishing. Theses beads take dye well, and a broad usable spectrum of bold colors is available. Unfortunately, these properties are widely misused to create a fake turquoise.

The downside of working with magnesite/periclase is the occupational risk. In the U.S., magnesite is listed as a recognized occupational hazard capable of causing respiratory problems or irritation of the eyes or skin. The immediate remedies are fresh air or irrigation of body parts.
The hazard is partly due to the raw magnesite itself, and partly due to its downstream industrial transformation to magnesium oxide (periclase, MgO). Ingestion of MgO results in ‘metal fume fever,’ a condition characterized by chest tightness, cough, aches, fever and chills, and a metallic taste in the mouth. Onset of symptoms can be delayed for several hours after exposure, and last for up to two days.
Magnesite Occurrence
Worldwide, estimated resources of magnesite are on the order of 10 billion tons. However, magnesite has minimum popularity among collectors. This attributes mainly to its dull colors and usually massive forms. Crystals are rare and also soft and brittle.

Probably the best magnesite crystals ever originate in several mines around Brumado, Bahia in Brazil, where they are found together with green or brown-red uvite (tourmaline). Nice Chinese specimens are from Shangbao, Hunan Province and Liaoning district, Liaoning Province.
Number of commercial deposits in Washington state, southwest of Chewelah, Stevens County; in California, at the Red Mountain District, Stanislaus and Santa Clara County; and in Nevada from the large Gabbs District deposit, Nye County. Nice specimens came from Hoboken, Hudson County, New Jersey and Staten Island, New York City, New York. Other occurrences which have been noted for good specimens include sites in Canada, in the Del Oro deposits, Timmins, Ontario and at the Mt. Brussilof mine, near Radium, British Columbia;
Magnesite has two co-type localities in Europe: Magnesia in Thessaly, Greece and Monti Pelati in Piedmont, Italy. Brown breunnerite crystals come from Vizze Valley (Val di Vizze), Bolzano, Italy. Nice magnesite crystals were found in Oberdorf an der Laming and other places in Styria, Austria.





Comments