Galena – Mineral Properties, Photos and Occurrence
Galena, historically also named lead glance, is among the most abundant sulfides. Besides forming aesthetic mineral specimens, galena is the primary lead ore. And galena itself was a first semiconductor ever used.
Crystal Structure of Galena
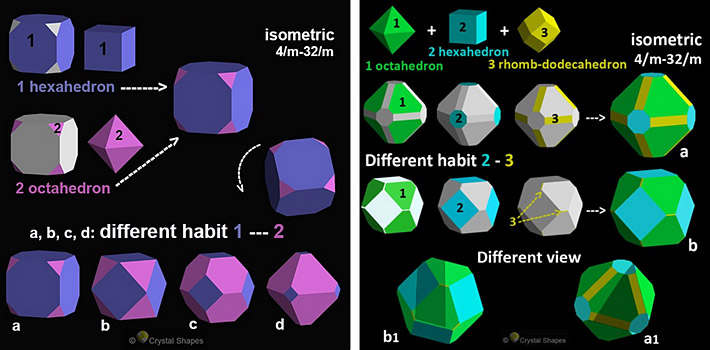
The chemical formula of galena is PbS (lead sulfide). It belongs to the isometric crystal system, most common crystal shapes are cubes, octahedrons, and their combinations. The dodecahedron crystal shapes are much less common, but over 40 galena crystal shapes are known. Twinning is common on {111} (spinel law twins). Skeletal, hopper or etched crystals are quite rare. Galena also forms massive, grainy, fibrous or platy aggregates.
Lead and sulfur atoms in galena crystal lattice form cubic units similar to the halite structure. The galena structure can accommodate small amounts of other metals such as copper, arsenic, bismuth, antimony, selenium, and most importantly, silver. Galena can contain as much as 20 wt.% of silver, but content from 0.1 wt.% to 1 wt.% is most common in so called argentiferous galena. Galena crystals are natural semiconductors and were the first material used in diodes.
Galena belongs to the sulfide class, more precisely its octahedral sulfide. Galena is the only common member of the Galena group, other minerals in this group are uncommon or rare. Galena makes a solid solution with clausthalite (PbSe), which is also member of galena group.
Though the mineral contains lead and regularly hits viral nonsense most toxic minerals lists, it is generally safe to handle. The lead sulfide is highly insoluble and does not react with skin. We cannot recommend licking or eating though, and heating galena is a very stupid idea because of the production of toxic fumes. Also avoid inhaling galena dust.
Physical Properties of Galena
Galena is a very soft mineral, registering only 2.5 on the Moh's hardness scale. And even worse, galena has perfect cleavage and brittle tenacity. This means that specimens are very easily damaged by just a tiny chip or bruise. When dropped or smashed, galena easily splits into tiny cubes. The fracture is subconchoidal, but very difficult to observe because of the perfect cleavage.

Fresh galena has shiny silver color, but it tarnishes quickly, turning lead grey and later dull grey. The galena streak is lead grey. The luster of fresh galena is brightly metallic, turning less bright to dull with tarnish.
The tarnish may be removed by simply cleaning with soap and water. However, the very low mechanical resistance and softness of galena make this always a risky operation.
The specific gravity of galena is about 7.6 g/cm3.
Origin
Deposits of galena are found in mid to lower temperature hydrothermal veins associated with igneous and metamorphic rocks. These veins may reach far into neighboring sedimentary layers. These veins are also known as polymetallic deposits because they usually contain also sphalerite (zinc ore), chalcopyrite (copper ore), pyrite, arsenopyrite and pyrrhotite.
Common associated gangue minerals include quartz, calcite, dolomite or fluorite.

Near-surface weathered parts of ore deposits (called gossan or iron cap) often contain significant amount of limonite with secondary minerals. Most of the lead bearing minerals originate by decomposition of galena, the most common being: cerussite, anglesite, pyromorphite, wulfenite, and less common like mimetite, vanadinite or crocoite.
Secondary galena can form in sediments with low oxygen content (reducing environment). Galena can also precipitate from hot fumes on the burning coal dumps or volcanic vents (fumaroles). Galena can form in some less common Pb-rich skarns or very rarely in pegmatites.
Industrial Applications
Galena is the primary ore of lead and may be found in a large variety of locations. Important even to early civilizations due to its relatively low melting point, it continues to be an often-mined mineral across the world.
Smelting galena can be as simple as throwing the mineral into a wood fire and collecting the cooled lead from beneath the ashes. Lead was used to create Roman plumbing networks, where the metal was known under its Latin name as plumbum, giving us the atomic symbol of Pb for lead.

Though galena is the primary ore of lead, argentiferous (silver-bearing) galena is an important silver ore. Deposits of Ag-rich galena are much more important economically. Though silver usually makes up <1% of galena weight, it generates much more revenue than lead.
Modern uses for galena include the use of galena crystals in earlier radio devices. It is of particular importance to the automotive, electronics and construction industries.
In automotive, lead is used in the production of lead-acid batteries for cars, trucks, and other motorized vehicles. It is also used to produce lead-bearing alloys for use in engine components, such as connecting rods and cylinder liners.

Lot of lead is used in the production of lead-based solders and lead-based components for a variety of electronics, including computers, mobile phones, and other electronic devices.
In construction, lead is used in the production of lead-based paints, sealants, and waterproofing materials. It is also used to produce lead-based roofing materials, such as shingles and flashing.
A big amount of lead is used in ammunition. Despite the current ban of lead fishing weights, because of their toxicity, the replacement of lead in ammunition is very difficult. Materials like bismuth alloys are very expensive and too brittle. And alloys like tombac (copper-zinc alloy with 5-20% zinc, also spelled tombak) are too light and too hard, which basically renders them highly imprecise, but with much greater piercing power.

Galena Occurrence and Notable Deposits
European localities include classic sites like Alston Moore and Weardale in England, Freiberg and Neudorf in Germany, Příbram and Stříbro in Czech Republic, Banská Štiavnica in Slovakia, Madan in Bulgaria, Herja and Turt in Romania, Trepča in Kosovo or ancient mines in Sardinia. Excellent banded aggregates of galena, sphalerite and marcasite (sometimes called schalenblende) occur in Olkusz in Poland, Segen Gottes Mine in Germany, and Schmalgraf Mine in Belgium.

Some of the most important galena deposits in North America are found in altered carbonate and cherts of the tri-state mining district of Upper Mississippi River Valley - the so called Lead Belt. Famous sites include Joplin and Reynolds in Missouri, Treece and Baxter Springs in Kansas and Pitcher in Oklahoma. There even exist towns called Galena in Missouri and Kansas. Other sites include Leadville, Empire and Silverton in Colorado, big lead mine is in Shullsburg, Wisconsin.

Other interesting localities include Sullivan mine in British Colombia, Broken Hill in Australia or Dalnegorsk in Russia. Many hydrothermal deposits are mined in Peru, Bolivia and Argentina. Tsumeb in Namibia and Oujda and Mibladen in Morocco are galena deposits with world class secondary minerals like cerussite, anglesite and many others.






Comments